Deoxyguanosine forms a bis-adduct with E,E-muconaldehyde, an oxidative metabolite of benzene: implications for the carcinogenicity of benzene
- PMID: 21972945
- PMCID: PMC3408037
- DOI: 10.1021/tx2002838
Deoxyguanosine forms a bis-adduct with E,E-muconaldehyde, an oxidative metabolite of benzene: implications for the carcinogenicity of benzene
Abstract
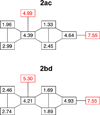
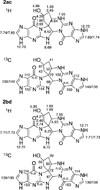
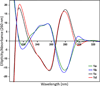
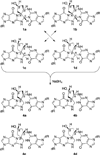
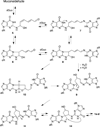
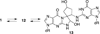
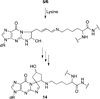
Benzene is employed in large quantities in the chemical industry and is an ubiquitous contaminant in the environment. There is strong epidemiological evidence that benzene exposure induces hematopoietic malignancies, especially acute myeloid leukemia, in humans, but the chemical mechanisms remain obscure. E,E-Muconaldehyde is one of the products of metabolic oxidation of benzene. This paper explores the proposition that E,E-muconaldehyde is capable of forming Gua-Gua cross-links. If formed in DNA, the replication and repair of such cross-links might introduce structural defects that could be the origin of the carcinogenicity. We have investigated the reaction of E,E-muconaldehyde with dGuo and found that the reaction yields two pairs of interconverting diastereomers of a novel heptacyclic bis-adduct having a spiro ring system linking the two Gua residues. The structures of the four diastereomers have been established by NMR spectroscopy and their absolute configurations by comparison of CD spectra with those of model compounds having known configurations. The final two steps in the formation of the bis-nucleoside (5-ring → 6-ring → 7-ring) have significant reversibility, which is the basis for the observed epimerization. The 6-ring precursor was trapped from the equilibrating mixture by reduction with NaBH(4). The anti relationship of the two Gua residues in the heptacyclic bis-adduct precludes it from being formed in B DNA, but the 6-ring precursor could readily be accommodated as an interchain or intrachain cross-link. It should be possible to form similar cross-links of dCyt, dAdo, the ε-amino group of lysine, the imidazole NH of histidine, and N termini of peptides with the dGuo-muconaldehyde monoadduct.
Figures









References
-
- Whysner J, Reddy MV, Ross PM, Mohan M, Lax EA. Genotoxicity of benzene and its metabolites. Mutat. Res. 2004;566:99–130. - PubMed
-
- Bayliss DL, Chen C, Jarabek A, Sonawane B, Valcovic L. U. S. Environmental Protection Agency; 1998. Carcinogenic effects of benzene: An update. EPA/600/P-97/001F http://www.epa.gov/ncea/pdfs/benzenef.pdf. - PubMed
-
- Bond JA, Rice JM, editors. Benzene 2009-Health effects and mechanisms of bone marrow toxicity: Implications for t-AML and the mode of action framework. Chem. Biol. Interact. 2010;184:1–312. - PubMed
-
- McMichael AJ. Carcinogenicity of benzene, toluene and xylene: epidemiological and experimental evidence. IARC Sci. Publ. 1988:3–18. - PubMed
-
- Henderson RF, Sabourin PJ, Medinsky MA, Birnbaum LS, Lucier GL. Benzene dosimetry in experimental animals: relevance for risk assessment. Prog. Clin. Biol. Res. 1992;374:93–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

