Pathophysiology, staging and therapy of severe sepsis in baboon models
- PMID: 21972970
- PMCID: PMC3263329
- DOI: 10.1111/j.1582-4934.2011.01454.x
Pathophysiology, staging and therapy of severe sepsis in baboon models
Abstract
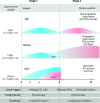
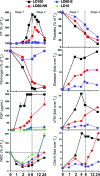
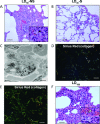
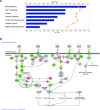
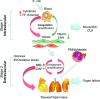
We review our baboon models of Escherichia coli sepsis that mimic, respectively, the shock/disseminated intravascular coagulation (DIC) and organ failure variants of severe sepsis, and analyse the pathophysiologic processes that are unique to each. The multi-stage, multi-factorial characteristics of severe sepsis develop as a result of the initial insult, which - depending on its intensity - activates components of the intravascular compartment leading to overwhelming shock/DIC; or initiates a sequence of events involving both the intra- and extravascular (tissues) compartments that lead to organ failure. In the latter case, the disorder passes through two stages: an initial inflammatory/coagulopathic intravascular first stage triggered by E. coli, followed by an extravascular second stage, involving components unique to each organ and triggered by ischemia/reperfusion (oxidative stress and histone release). Although a myriad of overlapping cellular and molecular components are involved, it is the context in which these components are brought into play that determine whether shock/DIC or organ failure predominate. For example, inflammatory and thrombotic responses amplified by thrombin in the first case whereas similar responses are amplified by complement activation products in the second. Rather than blocking specific mediators, we found that attenuation of the thrombin and complement amplification pathways can effectively reverse the shock/DIC and organ failure exhibited by the LD(100) and LD(50) E. coli models of severe sepsis, respectively. Translation of these concepts to successful intervention in the respective baboon models of E. coli sepsis and the application to their clinical counterparts is described.
© 2011 The Authors Journal of Cellular and Molecular Medicine © 2011 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures
References
-
- Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. - PubMed
-
- Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. - PubMed
-
- Taylor FB., Jr Staging of the pathophysiologic responses of the primate microvasculature to Escherichia coli and endotoxin: examination of the elements of the compensated response and their links to the corresponding uncompensated lethal variants. Crit Care Med. 2001;29:S78–89. - PubMed
-
- Taylor FB, Jr, Wada H, Kinasewitz G. Description of compensated and uncompensated disseminated intravascular coagulation (DIC) responses (non-overt and overt DIC) in baboon models of intravenous and intraperitoneal Escherichia coli sepsis and in the human model of endotoxemia: toward a better definition of DIC. Crit Care Med. 2000;28:S12–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

