Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer
- PMID: 21973086
- PMCID: PMC3265716
- DOI: 10.1111/j.1742-4658.2011.08385.x
Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer
Abstract
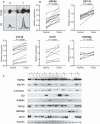
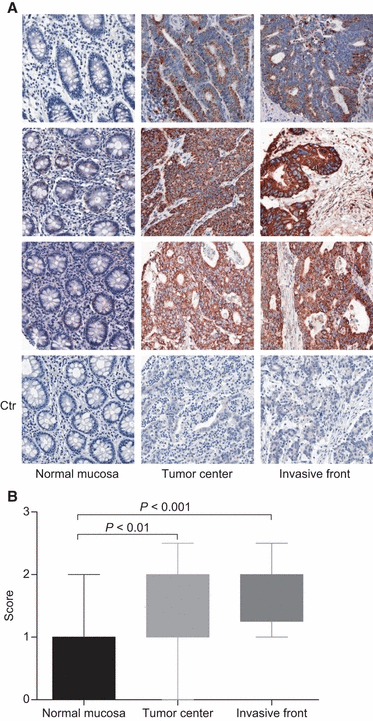
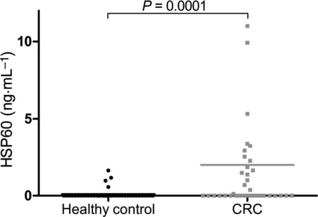
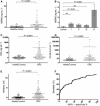
Colorectal cancer (CRC) is a major public health issue worldwide, and novel tumor markers may contribute to its efficient management by helping in early detection, prognosis or surveillance of disease. The aim of our study was to identify new serum biomarkers for CRC, and we followed a phased biomarker discovery and validation process to obtain an accurate preliminary assessment of potential clinical utility. We compared colonic tumors and matched normal tissue from 15 CRC patients, using two-dimensional difference gel electrophoresis (2D-DIGE), and identified 17 proteins that had significant differential expression. These results were further confirmed by western blotting for heat shock protein (HSP) 60, glutathione-S-transferase Pi, α-enolase, T-complex protein 1 subunit β, and leukocyte elastase inhibitor, and by immunohistochemistry for HSP60. Using mAbs raised against HSP60, we developed a reliable (precision of 5-15%) and sensitive (0.3 ng·mL(-1)) immunoassay for the detection of HSP60 in serum. Elevated levels of HSP60 were found in serum from CRC patients in two independent cohorts; the receiver-operating characteristic curve obtained in 112 patients with CRC and 90 healthy controls had an area under the curve (AUC) of 0.70, which was identical to the AUC of carcinoembryonic antigen. Combination of serum markers improved clinical performance: the AUC of a three-marker logistic regression model combining HSP60, carcinoembryonic antigen and carbohydrate antigen 19-9 reached 0.77. Serum HSP60 appeared to be more specific for late-stage CRC; therefore, future studies should evaluate its utility for determining prognosis or monitoring therapy rather than early detection.
© 2011 bioMérieux Journal compilation © 2011 FEBS.
Figures

References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Walsh JM, Terdiman JP. Colorectal cancer screening: clinical applications. JAMA. 2003;289:1297–1302. - PubMed
-
- Burt RW. Colorectal cancer screening. Curr Opin Gastroenterol. 2010;26:466–470. - PubMed
-
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. - PubMed
-
- Nelson DB, McQuaid KR, Bond JH, Lieberman DA, Weiss DG, Johnston TK. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc. 2002;55:307–314. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

