Cavitation clouds created by shock scattering from bubbles during histotripsy
- PMID: 21973343
- PMCID: PMC3206907
- DOI: 10.1121/1.3625239
Cavitation clouds created by shock scattering from bubbles during histotripsy
Abstract
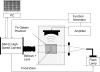
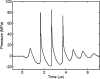
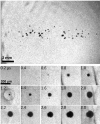
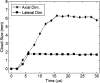
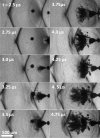
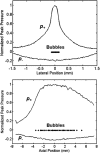
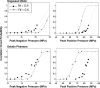
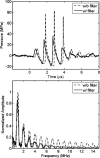
Histotripsy is a therapy that focuses short-duration, high-amplitude pulses of ultrasound to incite a localized cavitation cloud that mechanically breaks down tissue. To investigate the mechanism of cloud formation, high-speed photography was used to observe clouds generated during single histotripsy pulses. Pulses of 5-20 cycles duration were applied to a transparent tissue phantom by a 1-MHz spherically focused transducer. Clouds initiated from single cavitation bubbles that formed during the initial cycles of the pulse, and grew along the acoustic axis opposite the propagation direction. Based on these observations, we hypothesized that clouds form as a result of large negative pressure generated by the backscattering of shockwaves from a single bubble. The positive-pressure phase of the wave inverts upon scattering and superimposes on the incident negative-pressure phase to create this negative pressure and cavitation. The process repeats with each cycle of the incident wave, and the bubble cloud elongates toward the transducer. Finite-amplitude propagation distorts the incident wave such that the peak-positive pressure is much greater than the peak-negative pressure, which exaggerates the effect. The hypothesis was tested with two modified incident waves that maintained negative pressure but reduced the positive pressure amplitude. These waves suppressed cloud formation which supported the hypothesis.
© 2011 Acoustical Society of America
Figures



References
-
- Pishchalnikov Y. A., Sapozhnikov O. A., Bailey M. R., Williams J. C., Cleveland R. O., Colonius T., Crum L. A., Evan A. P., and McAteer J. A., “Cavitation bubble cluster activity in the breakage of kidney stones by lithotripter shockwaves,” J. Endourol. 17, 435–446 (2003). 10.1089/089277903769013568 - DOI - PMC - PubMed
-
- Evan A. P., Willis L. R., McAteer J. A., Bailey M. R., Connors B. A., Shao Y., Lingeman J. E., Williams J. C., Fineberg N. S., and Crum L. A., “Kidney damage and renal functional changes are minimized by waveform control that suppresses cavitation in shock wave lithotripsy,” J. Urol. 168, 1556–1562 (2002). 10.1016/S0022-5347(05)64520-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

