Oxidative damages in tubular epithelial cells in IgA nephropathy: role of crosstalk between angiotensin II and aldosterone
- PMID: 21974877
- PMCID: PMC3203061
- DOI: 10.1186/1479-5876-9-169
Oxidative damages in tubular epithelial cells in IgA nephropathy: role of crosstalk between angiotensin II and aldosterone
Abstract
Background: Inhibition of the renin-angiotensin-aldosterone system (RAAS) slows down the progression of chronic renal diseases (CKD) including IgA nephropathy (IgAN). Herein, we studied the pathogenetic roles of aldosterone (Aldo) in IgAN.
Methods: Human mesangial cells (HMC) was activated with polymeric IgA (pIgA) from IgAN patients and the effects on the expression of RAAS components and TGF-β synthesis examined. To study the roles of RAAS in the glomerulotubular communication, proximal tubular epithelial cells (PTEC) was cultured with conditioned medium from pIgA-activated HMC with eplerenone or PD123319, the associated apoptotic event was measured by the generation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and reactive oxygen species (ROS).
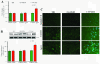
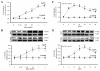
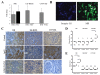
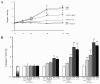
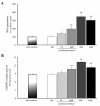
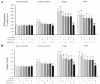
Results: Polymeric IgA up-regulated the Aldo synthesis and aldosterone synthase expression by HMC. The release of TGF-β by HMC was up-regulated synergistically by AngII and Aldo and this was inhibited by incubation of HMC with losartan plus eplerenone. Cultured PTEC express the mineralocorticoid receptor, but not synthesizing aldosterone. Apoptosis, demonstrated by cleaved PARP expression and caspase 3 activity, was induced in PTEC activated by conditioned medium prepared from HMC cultured with pIgA from IgAN patients. This apoptotic event was associated with increased generation of NADPH oxidase and ROS. Pre-incubation of PTEC with PD123319 and eplerenone achieved complete inhibition of PTEC apoptosis.
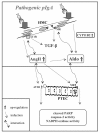
Conclusions: Our data suggest that AngII and Aldo, released by pIgA activated HMC, served as mediators for inducing apoptosis of PTEC in glomerulo-tubular communications. Crosstalk between AngII and Aldo could participate in determining the tubular pathology of IgAN.
Figures





References
-
- D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727. - PubMed
-
- Coppo R, Amore A, Gianoglio B, Cacace G, Picciotto G, Roccatello D, Peruzzi L, Piccoli G, De Filippi PG. Angiotensin II local hyperreactivity in the progression of IgA nephropathy. Am J Kidney Dis. 1993;21:593–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

