Thyroid hormone drives fetal cardiomyocyte maturation
- PMID: 21974928
- PMCID: PMC3250248
- DOI: 10.1096/fj.10-179895
Thyroid hormone drives fetal cardiomyocyte maturation
Abstract
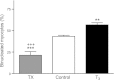
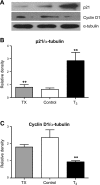
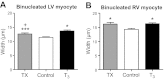
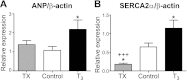
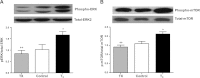
Tri-iodo-l-thyronine (T(3)) suppresses the proliferation of near-term serum-stimulated fetal ovine cardiomyocytes in vitro. Thus, we hypothesized that T(3) is a major stimulant of cardiomyocyte maturation in vivo. We studied 3 groups of sheep fetuses on gestational days 125-130 (term ∼145 d): a T(3)-infusion group, to mimic fetal term levels (plasma T(3) levels increased from ∼0.1 to ∼1.0 ng/ml; t(1/2)∼24 h); a thyroidectomized group, to produce low thyroid hormone levels; and a vehicle-infusion group, to serve as intact controls. At 130 d of gestation, sections of left ventricular freewall were harvested, and the remaining myocardium was enzymatically dissociated. Proteins involved in cell cycle regulation (p21, cyclin D1), proliferation (ERK), and hypertrophy (mTOR) were measured in left ventricular tissue. Evidence that elevated T(3) augmented the maturation rate of cardiomyocytes included 14% increased width, 31% increase in binucleation, 39% reduction in proliferation, 150% reduction in cyclin D1 protein, and 500% increase in p21 protein. Increased expression of phospho-mTOR, ANP, and SERCA2a also suggests that T(3) promotes maturation and hypertrophy of fetal cardiomyocytes. Thyroidectomized fetuses had reduced cell cycle activity and binucleation. These findings support the hypothesis that T(3) is a prime driver of prenatal cardiomyocyte maturation.
Figures
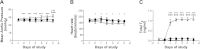

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

