New insights into human minimal change disease: lessons from animal models
- PMID: 21974967
- PMCID: PMC3253318
- DOI: 10.1053/j.ajkd.2011.07.024
New insights into human minimal change disease: lessons from animal models
Abstract
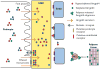
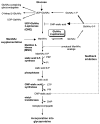
The pathogenesis of minimal change disease (MCD), considered to be the simplest form of nephrotic syndrome, has been one of the major unsolved mysteries in kidney disease. In this review, recent landmark studies that have led to the unraveling of MCD are discussed. A recent study now explains the molecular basis of major clinical and morphologic changes in MCD. Overproduction of angiopoietin-like 4 (ANGPTL4) in podocytes in MCD causes binding of ANGPTL4 to the glomerular basement membrane, development of nephrotic-range selective proteinuria, diffuse effacement of foot processes, and loss of glomerular basement membrane charge, but is not associated with changes shown by light microscopy in the glomerular and tubulointerstitial compartments. At least some of this ability of ANGPTL4 to induce proteinuria is linked to a deficiency of sialic acid residues because oral supplementation with sialic acid precursor N-acetyl-d-mannosamine improves sialylation of podocyte-secreted ANGPTL4 and significantly decreases proteinuria. Animal models of MCD, recent advances in potential biomarkers, and studies of upstream factors that may initiate glomerular changes also are discussed. In summary, recent progress in understanding MCD is likely to influence the diagnosis and treatment of MCD in the near future.
Copyright © 2012 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures

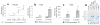

References
-
- Nachman PH, Jennette JC, Falk R. Primary glomerular disease. In: Brenner BM, editor. The Kidney. 8. Philadelphia, PA: Elsevier; 2008. pp. 987–1066.
-
- Miner JH. Glomerular filtration: the charge debate charges ahead. Kidney Int. 2008;74(3):259–261. - PubMed
-
- Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, et al. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296(2):F213–F229. - PubMed
-
- Seiler MW, Venkatachalam MA, Cotran RS. Glomerular epithelium: structural alterations induced by polycations. Science. 1975;189(4200):390–393. - PubMed
-
- Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS. Pathogenesis of polycation-induced alterations (“fusion”) of glomerular epithelium. Lab Invest. 1977;36(1):48–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

