Versatility of PRMT5-induced methylation in growth control and development
- PMID: 21975038
- PMCID: PMC3225484
- DOI: 10.1016/j.tibs.2011.09.001
Versatility of PRMT5-induced methylation in growth control and development
Abstract
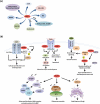
Arginine methylation governs important cellular processes that impact growth and proliferation, as well as differentiation and development. Through their ability to catalyze symmetric or asymmetric methylation of histone and non-histone proteins, members of the protein arginine methyltransferase (PRMT) family regulate chromatin structure and expression of a wide spectrum of target genes. Unlike other PRMTs, PRMT5 works in concert with a variety of cellular proteins including ATP-dependent chromatin remodelers and co-repressors to induce epigenetic silencing. Recent work also implicates PRMT5 in the control of growth-promoting and pro-survival pathways, which demonstrates its versatility as an enzyme involved in both epigenetic regulation of anti-cancer target genes and organelle biogenesis. These studies not only provide insight into the molecular mechanisms by which PRMT5 contributes to growth control, but also justify therapeutic targeting of PRMT5.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

References
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. - PubMed
-
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. - PubMed
-
- Berger SL. Histone modifications in transcriptional regulation. Current opinion in genetics & development. 2002;12:142–148. - PubMed
-
- Bedford MT. Arginine methylation at a glance. Journal of cell science. 2007;120:4243–4246. - PubMed
-
- Pal S, Sif S. Interplay between chromatin remodelers and protein arginine methyltransferases. Journal of cellular physiology. 2007;213:306–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

