DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials
- PMID: 21975270
- PMCID: PMC7185472
- DOI: 10.1016/S1473-3099(11)70240-7
DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials
Abstract
Background: Because the general population is largely naive to H5N1 influenza, antibodies generated to H5 allow analysis of novel influenza vaccines independent of background immunity from previous infection. We assessed the safety and immunogenicity of DNA encoding H5 as a priming vaccine to improve antibody responses to inactivated influenza vaccination.
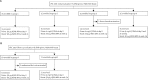
Methods: In VRC 306 and VRC 310, two sequentially enrolled phase 1, open-label, randomised clinical trials, healthy adults (age 18-60 years) were randomly assigned to receive intramuscular H5 DNA (4 mg) at day 0 or twice, at day 0 and week 4, followed by H5N1 monovalent inactivated vaccine (MIV; 90 μg) at 4 or 24 weeks, and compared with a two-dose regimen of H5N1 MIV with either a 4 or 24 week interval. Antibody responses were assessed by haemagglutination inhibition (HAI), ELISA, neutralisation (ID(80)), and immunoassays for stem-directed antibodies. T cell responses were assessed by intracellular cytokine staining. After enrolment, investigators and individuals were not masked to group assignment. VRC 306 and VRC 310 are registered with ClinicalTrials.gov, numbers NCT00776711 and NCT01086657, respectively.
Findings: In VRC 306, 60 individuals were randomly assigned to the four groups (15 in each) and 59 received the vaccinations. In VRC 310, of the 21 individuals enrolled, 20 received the vaccinations (nine received a two-dose regimen of H5N1 MIV and 11 received H5 DNA at day 0 followed by H5N1 MIV at week 24). H5 DNA priming was safe and enhanced H5-specific antibody titres following an H5N1 MIV boost, especially when the interval between DNA prime and MIV boost was extended to 24 weeks. In the two studies, DNA priming with a 24-week MIV boost interval induced protective HAI titres in 21 (81%) of 26 of individuals, with an increase in geometric mean titre (GMT) of more than four times that of individuals given the MIV-MIV regimen at 4 or 24 weeks (GMT 103-206 vs GMT 27-33). Additionally, neutralising antibodies directed to the conserved stem region of H5 were induced by this prime-boost regimen in several individuals. No vaccine-related serious adverse events were recorded.
Interpretation: DNA priming 24 weeks in advance of influenza vaccine boosting increased the magnitude of protective antibody responses (HAI) and in some cases induced haemagglutinin-stem-specific neutralising antibodies. A DNA-MIV vaccine regimen could enhance the efficacy of H5 or other influenza vaccines and shows that anti-stem antibodies can be elicited by vaccination in man.
Funding: National Institutes of Health.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
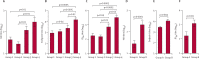


Comment in
-
Two is better than one.Lancet Infect Dis. 2011 Dec;11(12):889-91. doi: 10.1016/S1473-3099(11)70256-0. Epub 2011 Oct 3. Lancet Infect Dis. 2011. PMID: 21975267 No abstract available.
References
-
- WHO . Influenza (seasonal) fact sheet. World Health Organization; Geneva, Switzerland: 2009.
-
- Bastien N, Antonishyn NA, Brandt K. Human infection with a triple-reassortant swine influenza A(H1N1) virus containing the hemagglutinin and neuraminidase genes of seasonal influenza virus. J Infect Dis. 2010;201:1178–1182. - PubMed
-
- Shinde V, Bridges CB, Uyeki TM. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med. 2009;360:2616–2625. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

