Adjuvant chemotherapy for endometrial cancer after hysterectomy
- PMID: 21975736
- PMCID: PMC4164379
- DOI: 10.1002/14651858.CD003175.pub2
Adjuvant chemotherapy for endometrial cancer after hysterectomy
Abstract
Background: Endometrial adenocarcinoma (womb cancer) is a malignant growth of the lining (endometrium) of the womb (uterus). It is distinct from sarcomas (tumours of the uterine muscle). Survival depends the risk of microscopic metastases after surgery. Adjuvant (postoperative) chemotherapy improves survival from some other adenocarcinomas, and there is evidence that endometrial cancer is sensitive to cytotoxic therapy. This systematic review examines the effect of chemotherapy on survival after hysterectomy for endometrial cancer.
Objectives: To assess efficacy of adjuvant (postoperative) chemotherapy for endometrial cancer.
Search strategy: We searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library 2010, Issue 3), MEDLINE and EMBASE up to August 2010, registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria: Randomised controlled trials (RCTs) comparing adjuvant chemotherapy with any other adjuvant treatment or no other treatment.
Data collection and analysis: We used a random-effects meta-analysis to assess hazard ratios (HR) for overall and progression-free survival and risk ratios (RR) to compare death rates and site of initial relapse.
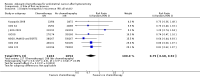
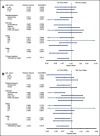
Main results: Five RCTs compared no additional treatment with additional chemotherapy after hysterectomy and radiotherapy. Four trials compared platinum based combination chemotherapy directly with radiotherapy. Indiscriminate pooling of survival data from 2197 women shows a significant overall survival advantage from adjuvant chemotherapy (RR (95% CI) = 0.88 (0.79 to 0.99)). Sensitivity analysis focused on trials of modern platinum based chemotherapy regimens and found the relative risk of death to be 0.85 ((0.76 to 0.96); number needed to treat for an additional beneficial outcome (NNT) = 25; absolute risk reduction = 4% (1% to 8%)). The HR for overall survival is 0.74 (0.64 to 0.89), significantly favouring the addition of postoperative platinum based chemotherapy. The HR for progression-free survival is 0.75 (0.64 to 0.89). This means that chemotherapy reduces the risk of being dead at any censorship by a quarter. Chemotherapy reduces the risk of developing the first recurrence outside the pelvis (RR = 0.79 (0.68 to 0.92), 5% absolute risk reduction; NNT = 20). The analysis of pelvic recurrence rates is underpowered but the trend suggests that chemotherapy may be less effective than radiotherapy in a direct comparison (RR = 1.28 (0.97 to 1.68)) but it may have added value when used with radiotherapy (RR = 0.48 (0.20 to 1.18)).
Authors' conclusions: Postoperative platinum based chemotherapy is associated with a small benefit in progression-free survival and overall survival irrespective of radiotherapy treatment. It reduces the risk of developing a metastasis, could be an alternative to radiotherapy and has added value when used with radiotherapy.
Conflict of interest statement
Nck Johnson, Andrew Bryant and Tracie Miles have no conflicts of interest.
Thomas Hogberg has participated in advisory boards for Bristol Myers Squibb and Bayer Schering Pharma
Figures









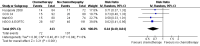
























Update of
References
References to studies included in this review
EORTC 55991 {unpublished data only}
-
- Kristensen G. A randomized trial of adjuvant treatment with radiation plus chemotherapy versus radiation alone in high risk endometrial carcinoma [EORTC 55991]. http://groups.eortc.be/gcg/studyprotocols.htm#55991. http://groups.eortc.be/gcg/studyprotocols.htm#55991.
GICOG {published data only}
-
- Maggi R, Cagnazzo G, Atlante G, Marinaccio M. Risk groups and adjuvant therapy in surgical staged endometrial cancer patients. A randomized multicentre study comparing chemotherapy with radiation therapy. International Journal of Gynecological Cancer. 1999; Vol. 99;9 Suppl 1:85 Abstract B73.
-
- Maggi R, Cucchi L, Restelli C, Delzotti F, Frigoli A. Post surgical treatment of endometrial cancer [GICOG trial]. International Journal of Gynecological Cancer 2005;93(3 Suppl 1):46, Abstract 166.
GOG 122 {published and unpublished data}
-
- Bruner DW, Barsevick A, Tian C, Randall M, Mannel R, Cohn DE, et al. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Quality of Life Research: an international journal of quality of life aspects of treatment, care and rehabilitation 2007;16(1):89‐100. - PubMed
-
- Randall ME. ASCO media player [Whole abdominal radiotherapy versus combination doxorubicin‐cisplatin chemotherapy in advanced endometrial carcinoma: A randomized phase III trial of the Gynecologic Oncology Group]. http://media.asco.org/asco/meetings_education/vm/2003/slides_only/frame.... abdominal radiotherapy versus combination doxorubicin‐cisplatin chemotherapy in advanced endometrial carcinoma: A randomized phase III trial of the Gynecologic Oncology Group&Speaker=Marcus E Randall, MD&PlayThumb=0&SpeakerName=Presenter%3A%20Marcus%20E%20Randall%2C%20MD&LectureName=Whole%20abdominal%20radiotherapy%20versus%20combination%20doxorubicin‐cisplatin%20chemotherapy%20in%20advanced%20endometrial%20carcinoma%3A%20A%20randomized%20phase%20III%20trial%20of%20the%20Gynecologic%20Oncology%20Group%20&Key=vm_23_5_353_1896&SessionName=Plenary%20Session&mediaURL=/media&ServerName=media.asco.org, 2003.
-
- Randall ME, Brunetto G, Muss H, Mannel RS, Spirtos N, Jeffrey F, Thigpen JT, Benda J. Whole abdominal radiotherapy versus combination doxorubicin‐cisplatin chemotherapy in advanced endometrial carcinoma: A randomized phase III trial of the Gynecologic Oncology Group. Proceedings of the American Society of Clinical Oncology. 2003; Vol. 22:2. Abstract 3.
-
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized Phase III Trial of Whole‐Abdominal Irradiation Versus Doxorubicin and Cisplatin Chemotherapy in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2006;24(1):36‐44. - PubMed
-
- Thomas G. ASCO media player [Plenary Session (Dr Thomas critiquing GOG 122)]. http://media.asco.org/asco/meetings_education/vm/2003/slides_only/frame.... Session&Speaker=Gillian Thomas, MD&PlayThumb=0&SpeakerName=Discussant%3A%20Gillian%20Thomas%2C%20MD&LectureName=A%20Randomized%20Trial%20of%20WAI%20vs.%20A%2FP%20in%20Advanced%20Endometrial%20CA&Key=vm_23_5_353_3585&SessionName=Plenary%20Session&mediaURL=/media&ServerName=media.asco.org. ASCO, 2003; Vol. http://media.asco.org/asco/meetings_education/module/video/frame.asp?eve....
GOG 150 {published data only}
-
- GOG Protocol FAQ. http://www.gog.org/protocolfaq.html.
-
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin‐ifosfamide and mesna (CIM) as post‐surgical therapy in stage I‐IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology 2007;107(2):177‐85. - PMC - PubMed
GOG 34 {published data only}
-
- Morrow CP, Bundy B, Creasman W, Homesley H. A randomized study of adriamycin adjuvant chemotherapy for patients with high‐risk stage I and II (occult) endometrial carcinoma: a GOG study. Proceedings, International Gynecological Cancer Society: Amsterdam, the Netherlands. 1987:87.
-
- Morrow CP, Bundy BN, Homesley HD, Creasman WT, Hornback NB, Kurman R, et al. Doxorubicin as an adjuvant following surgery and radiation therapy in patients with high‐risk endometrial carcinoma, stage I and occult stage II: A Gynecologic Oncology Group Study. Gynecologic Oncology 1990;36(2):166‐71. - PubMed
J GOG 2033 {published data only}
-
- Sagae S, Udagawa Y, Susumu N, Niwa K, Kudo R, Nozawa S. JGOG2033: Randomized phase III trial of whole pelvic radiotherapy vs cisplatin‐based chemotherapy in patients with intermediate risk endometrial carcinoma. Proceedings of the American Society of Clinical Oncology.2005: 41st Annual Meeting of the American Society of Clinical Oncology, May 13‐17, 2005. Orlando, Florida, 2005; Vol. Abstract 5002, issue http://media.asco.org/player/default.aspx?LectureID=1646&conferenceF....
-
- Susumu N, Sagae S, Udagawa Y, Niwa K, Kuramoto H, Satoh S, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin‐based combined chemotherapy in patients with intermediate‐ and high‐risk endometrial cancer: a Japanese Gynecologic Oncology Group study. Gynecologic Oncology 2008;108(1):226‐33. - PubMed
Kuoppala 2008 {published data only}
-
- Kuoppala T, Maenpaa J, Tomas E, Puistola U, Salmi T, Grenman S, et al. Surgically staged high‐risk endometrial cancer: randomized study of adjuvant radiotherapy alone vs. sequential chemo‐radiotherapy. Gynecol Oncol 2008;110(2):190‐5. - PubMed
MaNGO {unpublished data only}
NSGO {published and unpublished data}
-
- Hogberg T. Case #4 Endometrial CarcinomaStage IIIC. http://www.primeoncology.org/Files/Doc/SLIDES/EPGM06282008/Case4.pdf. 2008.
-
- Hogberg T, Rosenberg P, Kristensen G, Oliveira CF, Pont Christensen R, Sorbe B, et al. A randomized phase‐III study on adjuvant treatment with radiation (RT) ± chemotherapy (CT) in early‐stage high‐risk endometrial cancer. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Abstracts of the 43rd Annual Meeting of the American Society of Clinical Oncology; 1‐5 June 2007; Chicago, Illinois. www.asco.org, 2007; Vol. Part I.2007;25 (18 Suppl):Abstract 5503.
-
- Hogberg, T. [Presentation: A randomized phase‐III study on adjuvant treatment with radiation (RT) 177; chemotherapy (CT) in early‐stage high‐risk endometrial cancer (NSGO‐EC‐9501/EORTC 55991)]. ASCO media player, Oral Presentation Gynecologic Cancer Track. 2007; Vol. Abstract No: 5503:http://media.asco.org/player/default.aspx?LectureID=3418&conferenceF...,.
NSGO, MaNGO and EORTC {published data only}
NSGO & EORTC {unpublished data only}
-
- Kristensen G. Endometrial cancer [33rd ESMO Congress]. http://www.esmo.org/fileadmin/media/presentations/977/1739/Endometrial%2.... Stockholm, 2006.
References to studies excluded from this review
Aapro 2003 {published data only}
-
- Aapro MS, Wijk FH, Bolis G, Chevallier B, Burg MEL, Poveda A et al on behalf of the EORTC‐GCCG. Doxorubicin versus doxorubicin and cisplatin in endometrial carcinoma: definitive results of a randomised study (55872) by the EORTC‐GCCG. Annals of Oncology 2003;14:441‐8. - PubMed
Bruner 2007 {published data only}
-
- Bruner DW, Barsevick A, Tian C, Randall M, Mannel R, Cohn DE, et al. Randomized trial results of quality of life comparing whole abdominal irradiation and combination chemotherapy in advanced endometrial carcinoma: A gynecologic oncology group study. Quality of Life Research 2007;16(1):89‐100. - PubMed
Brunetto 2000 {published data only}
-
- Brunetto V, Kilgore L, Soper J, McGehee R, Olt G, Lentz SS, et al. A phase III trial of ifosfamide alone or in combination with cisplatin in the treatment of advanced, persistent or recurrent carcinosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology 2000;98;68(1):137, Abstract 266.
Chauvergne 2008 {published data only}
-
- Chauvergne J, Cappelaere P, Durand M, Brunet R, Hoerni B. Chemotherapy of cervico‐uterin carcinoma by two associations:cisplatine or detorubicine. Results of a comparative controlled trial. Bulletin du cancer 2008;81;68(2):363.
Cohen 1984 {published data only}
-
- Cohen CJ, Bruckner HW, Deppe G, Blessing JA, Homesley H, Lee JH, et al. Multidrug treatment of advanced and recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Obstetrics and Gynecology 1984;63(5):719‐26. - PubMed
Deng 2000 {published data only}
-
- Deng XH, Duan W, Li BZ. Estramustine phosphate in the treatment of endometrium carcinoma. Chinese Journal of Cancer Research 2000;12(1):58‐63.
Edmonson 1987 {published data only}
-
- Edmonson JH, Krook JE, Hilton JF, Malkasian GD, Everson LK, Jefferies JA, et al. Randomized phase II studies of cisplatin and a combination of cyclophosphamide‐doxorubicin‐cisplatin (CAP) in patients with progestin‐refractory advanced endometrial carcinoma. Gynecologic Oncology 1887;28(1):20‐4. - PubMed
Fleming 2004a {published data only}
-
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of Doxorubicin Plus Cisplatin With or Without Paclitaxel Plus Filgrastim in Advanced Endometrial Carcinoma: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2004;22(11):2159‐66. - PubMed
Fleming 2004b {published data only}
-
- Fleming GF, Filiaci VL, Bentley RC, Herzog T, Vaccarello L, Gallion H. Phase III randomised trial of doxorubicin + cisplatin versus doxorubicin + 24‐h paclitaxel + filgrastim in ednometrial carcinoma: a Gynecologic Oncology Group study. Annals of Oncology 2004;15:1173‐8. - PubMed
Fujimura 2000 {published data only}
-
- Fujimura H, Kikkawa F, Oguchi H, Nakashima N, Mizutani S. Adjuvant chemotherapy including cisplatin in endometrial carcinoma. Gynecologic & Obstetric Investigation 2000;50(2):127‐32. - PubMed
Gallion 2003 {published data only}
-
- Gallion HH, Brunetto VL, Cibull M, Lentz SS, Reid G, Soper JT, et al. Randomized phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrence endometrial carcinaoma: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2003;21(20):3808‐13. - PubMed
GCSF {published data only}
-
- Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2004;22(11):2159‐66. - PubMed
GOG 107 {published data only}
-
- Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. Journal of Clinical Oncology 2004;22(19):3902‐8. - PubMed
GOG 156 {unpublished data only}
-
- GOG Protocol FAQ. http://www.gog.org/protocolfaq.html.
GOG 161 {published data only}
-
- Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2007;25(5):526‐31. - PubMed
GOG 194 {unpublished data only}
-
- GOG Protocol FAQ. http://www.gog.org/protocolfaq.html.
-
- Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T, et al. Preliminary analysis of RTOG 9708: Adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high‐risk endometrial cancer. International Journal of Radiation Oncology, Biology, Physics. 2004;59(1):168‐73. - PubMed
Horton 1978 {published data only}
-
- Horton J, Begg CB, Arseneault J, Bruckner H, Creech R, Hahn RG. Comparison of adriamycin with cyclophosphamide in patients with advanced endometrial cancer. Cancer Treatment Report 1978;62(1):159‐61. - PubMed
Long 1995 {published data only}
-
- Long HJ, Nelimark RA, Cha SS. Comparison of methotrexate, vinblastine, doxorubicin and cisplatin (MVAC) vs doxorubicin and cisplatin (AC) in advanced endometrial carcinoma (Meeting abstract). Proceedings of the Annual Meeting of the American Society of Clinical Oncology. 1995; Vol. 14:282, Abstract 797.
Omura {published data only}
-
- Omura GA, Blessing JA, Major F, Silverberg S. A randomized trial of adriamycin versus no adjuvant chemotherapy in stage I and II uterine sarcomas. Proceedings of the American Society of Clinical Oncology. 1985; Vol. 83 (2):149, Abstract C‐580.
Pawinski 1999 {published data only}
-
- Pawinski A, Tumolo S, Hoesel G, Cervantes A, Oosterom AT, Hoctin Boes G, et al. Cyclophosphamide or ifosfamide in patients with advanced and/or recurrent endometrial carcinoma: a randomised phaseII study of the EORTC Gynecological Cancer Cooperative Group. European Journal of Obstetrics and Gynecology and Reproductive Biology 1999;86:179‐83. - PubMed
Samuels 2004 {published data only}
-
- Samuels BL, Rushing D, Chawla SP, Schuetze SM, Mehren M, Keohan ML, et al. Randomized phase II study of trabectedin (ET‐743) given by two different dosing schedules in patients with leiomyosarcomas or liposarcomas refractory to conventional doxorubicin and ifosfamide chemotherapy. Proceedings of the American Society of Clinical Oncology. 2004; Vol. 23:814, Abstract 9000..
Sutton 2000 {published data only}
-
- Sutton G, Brunetto VL, Kilgore L, Soper JT, McGehee R, Olt G, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology 2000;79(2):147‐53. - PubMed
Thigpen 1994 {published data only}
-
- Thigpen JT, Blessing JA, DiSaia PJ, Yordan E, Carson LF, Evers C. A randomized comparison of doxorubicin alone versus doxorubicin plus cyclophosphamide in the management of advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Journal of Clinical Oncology 1994;12(7):1408‐14. - PubMed
Thigpen 2004 {published data only}
-
- Thigpen JT, Brady MF, Homesley HD, Malfetano J, DuBeshter B, Burger RA, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: A Gynecologic Oncology Group Study (GOG). Journal of Clinical Oncology 2004;22(19):3902‐8. - PubMed
References to ongoing studies
GOG 249 {unpublished data only}
-
- Pelvic Radiation Therapy or Vaginal Implant Radiation Therapy, Paclitaxel, and Carboplatin in Treating Patients With High‐Risk Stage I or Stage II Endometrial Cancer. http://clinicaltrials.gov/ct2/show/NCT00807768.
GOG 258 {unpublished data only}
-
- GOG 0258 (RTOG 1073 Endorsed). A Randomized Phase III Trial of Cisplatin and Tumor Volume Directed Irradiation Followed by Carboplatin andPaclitaxel vs. Carboplatin and Paclitaxel for Optimally Debulked, Advanced Endometrial Carcinoma. http://www.rtog.org/LinkClick.aspx?fileticket=7aqw‐i1bTZ8%3D&tabid=331.
Hogberg {unpublished data only}
-
- Thomas Hogberg, MMC‐08‐03‐060, NCT01041027. Is the sequential combination of RT after four courses of CT better then 2 additional courses of CT in the adjuvant therapy of high‐risk endometrial cancer. A Phase III intergroup trial on adjuvant therapy in radically operated endometrial cancer patients with high risk for micro‐metastatic disease: 4 courses of adjuvant CT (CT) followed by radiation therapy (RT) versus 2 more courses of CT.
PORTEC 3 {unpublished data only}
-
- CKTO‐2006‐04ISRCTN14387080, CKTO‐PORTEC‐3, EU‐20664, NCT00411138. Phase III Randomized Study of Concurrent Chemoradiotherapy Followed By Adjuvant Chemotherapy Versus Pelvic Radiotherapy Alone in Patients With High‐Risk Stage IB‐III Endometrial Carcinoma Alternate Title. Chemotherapy and Radiation Therapy Compared With Radiation Therapy Alone in Treating Patients With High‐Risk Stage I, Stage II, or Stage III Endometrial Cancer.
Additional references
Abu‐Rustum 2010
Amant 2007
-
- Amant F, Moerman P, Neven P, Timmerman D, Limbergen E, Vergote I. Treatment modalities in endometrial cancer. Current Opinion in Oncology 2007;19(5):479‐85. - PubMed
Aoki 2004
-
- Aoki Y, Watanabe M, Amikura T. Adjuvant chemotherapy as treatment of high‐risk stage I and II endometrial cancer. Gynecologic Oncology 2004;94:333‐9. - PubMed
Butler 2004
-
- Butler L, Bacon M, Carey M, Zee B, Tu D, Bezjak A. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. Determining the relationship between toxicity and quality of life in an ovarian cancer chemotherapy clinical trial. Journal of Clinical Oncology 2004;15(22):2461‐8. - PubMed
Cheong 2007
-
- Cheong KA, Chrystal K, Harper PG. Adjuvant chemotherapy in non‐small cell lung cancer. International Journal of Clinical Practice 2007;611(1):143‐6. - PubMed
Cochrane Gynaecological Cancer Group
-
- Williams C, Quinn G, Jess C, Bishop T, Hayes J. Cochrane Gynaecological Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs) [Art. No.: GYNAECA]. Cochrane Database of Systematic Reviews 2010, issue 2. [http://www.mrw.interscience.wiley.com/cochrane/clabout/articles/GYNAECA/]
Creutzberg 2010
-
- Creutzberg C. Adjuvant Chemotherapy for Endometrial Cancer. International Journal of Gynecological Cancer 2010;20(11):S60‐S63. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). . BMJ Publication Group, 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Fehr 2006
-
- Fehr MK, Streich MS. The validity of chemotherapy in the treatment of endometrial cancer (German). Gynakologisch‐Geburtshilfliche Rundschau 2006;46(1‐2):34‐8. - PubMed
Ferlay 2004
-
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide IARC CancerBase No. 5, version 2.0. Lyon: IARCPress, 2004.
Ferlay 2010
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008 [GLOBOCAN 2008]. International Journal of Cancer 2010;127(12):2893‐917. - PubMed
Figueredo 2008
Gadducci 2007
-
- Gadducci A, Tana R, Cosio S, Genazzani AR. Adjuvant treatment in endometrial cancer. Ginecologia y Obstetricia de Mexico 2007;3(1):1‐7.
Galaal 2010
Gelber 1995
-
- Gelber RD, Cole BF, Goldhirsch A, Bonadonna G, Howell A, McArdle CS, et al. Adjuvant chemotherapy for premenopausal breast cancer: a meta‐analysis using quality‐adjusted survival. Cancer Journal from Scientific American 1995;1(2):114‐21. - PubMed
GOG 108
-
- Sutton GP, Blessing JA, Barrett RJ, Soper JT, McGehee R, Olt G, et al. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. American Journal of Obstetrics and Gynecology 1992;166(2):556‐9. - PubMed
GOG 9001
-
- Reisinger SA, Asbury R, Liao SY, Homesley HD. A phase I study of weekly cisplatin and whole abdominal radiation for the treatment of stage III and IV endometrial carcinoma. Gynecologic Oncology 1996;63(3):299‐303. - PubMed
GOG 94
-
- Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley HD, Malfetano JH, et al. Whole abdominal radiotherapy in the adjuvant treatment of patients with stage III and IV endometrial cancer: a gynecologic oncology group study. Gynecologic Oncology 2005;97(3):755‐63. - PubMed
Green 2005
Hayakawa 1995
-
- Hayakawa S, Sato S, Takano T, Wagazuma S, Yajima A. Chemotherapy of uterine endometrial cancer [Japanese]. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1995;22(9):1169‐75. - PubMed
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration. 2008.
Hirai 2002
-
- Hirai M, Hirono M, Oosaki T. Adjuvant chemotherapy in stage I uterine endometrial carcinoma. International Journal of Gynaecology and Obstetrics 2002;78:37‐44. - PubMed
Hogberg 2008
-
- Hogberg T. Adjuvant Chemotherapy in Endometrial Carcinoma: Overview of Randomised Trials. Clinical Oncology 2008;20(6):463‐9. - PubMed
Humber 2005
IOG (UK)
-
- UK‐improving outcomes guidance. Guidance on Commissioning Cancer Services Improving Outcomes in Gynaecological Cancers The Manual. http://www.cancercare.on.ca/common/pages/UserFile.aspx?serverId=6&pa.... Vol. Catalogue number 16149, London SE1 8UG: NHS Executive Health Services Directorate Wellington House London SE1 8UG. July 1999. NHS Executive Health Services Directorate Wellington House, July 1999..
Johnson 2007
-
- Johnson N, Cornes P. Survival and recurrent disease after postoperative radiotherapy for early endometrial cancer (systematic review and meta‐analysis). BJOG 2007;141(11):1313‐20. - PubMed
Kodama 2007a
-
- Kodama J, Seki N, Ojima Y. Efficacy and prognostic implications of administering adjuvant chemotherapy to patients with endometrial cancer that is confined to the uterus. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2007;131(1):76‐80. - PubMed
Kodama 2007b
-
- Hayakawa S, Sato S, Takano T, Wagazuma S, Yajima A. Chemotherapy of uterine endometrial cancer. Gan to Kagaku Ryoho [Japanese Journal of Cancer & Chemotherapy] 1995;95;22(9):1169‐75. - PubMed
Kong 2007
Lee 2006
-
- Lee CM, Szabo A, Shrieve DC, Macdonald OK, Gaffney DK. Frequency and Effect of Adjuvant Radiation Therapy Among Women With Stage I Endometrial Adenocarcinoma. JAMA 2006;295:389‐97. - PubMed
Lukka 2006
-
- Lukka H, Chambers A, Fyles A, Thephamongkhol K, Elit L, Fung‐Kee‐Fung M, et al. Adjuvant Radiotherapy in Women with Stage I Endometrial Cancer: A Clinical Practice Guideline. A Quality Initiative of the Program in Evidence‐based Care (PEBC) [Cancer Care Ontario Report]. http://www.cancercare.on.ca/common/pages/UserFile.aspx?serverId=6&pa... March 9, 2006.
Luoma 2002
-
- Luoma ML, Hakamies‐Blomqvist L, Sjöström J, Mouridsen H, Pluzanska A, Malmström P, et al. Physical performance, toxicity, and quality of life as assessed by the physician and the patient. Acta Oncologica 2002;41(1):44‐9. - PubMed
Martin‐Hirsch 1999
May 2010
Miller 2008
National Cancer Institute, USA
-
- National Cancer Institute, USA. http://www.cancer.gov/.
National Comprehensive Cancer Network
-
- National Comprehensive Cancer Network. http://www.nccn.org/clinical.asp.
Obel 2006
-
- Obel JC, Friberg G, Fleming GF. Chemotherapy in endometrial cancer. Clinical Advances in Hematology & Oncology 2006;4(6):459‐68. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Piver 2006
-
- Piver MS, Lele SB, Marchetti DL, Emrich LJ. Effect of adjuvant chemotherapy on time to recurrence and survival of stage I uterine sarcomas. Journal of Surgical Oncology 2006;38(4):233‐9. - PubMed
Rodriguez 2008
-
- Rodriguez AO. Chemotherapy for early stage endometrial cancer? Should we be using it?. Current Opinion in Obstetrics & Gynecology 2008;20(1):1‐3. - PubMed
Sobin 2009
-
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th Edition. Wiley‐Blackwell, 2009.
Stanojevic 2008
-
- Stanojevic Z, Djordjevic B, Todorovska I, Lilic V, Zivadinovic R, Dunjic O. Risk factors and adjuvant chemotherapy in the treatment of endometrial cancer. Journal of B.U.ON 2008;13(1):23‐30. - PubMed
Thomas 2003 [Computer program]
-
- Thomas G. ASCO media player [Plenary Session (Dr Thomas critiquing GOG 122); A Randomized Trial of WAI vs. A/P in Advanced Endometrial CA.]. http://media.asco.org/asco/meetings_education/vm/2003/slides_only/frame.... Session&Speaker=Gillian Thomas, MD&PlayThumb=0&SpeakerName=Discussant%3A%20Gillian%20Thomas%2C%20MD&LectureName=A%20Randomized%20Trial%20of%20WAI%20vs.%20A%2FP%20in%20Advanced%20Endometrial%20CA&Key=vm_23_5_353_3585&SessionName=Plenary%20Session&mediaURL=/media&ServerName=media.asco.org. ASCO, 2003; Vol. http://media.asco.org/asco/meetings_education/module/video/frame.asp?eve....
Tierney 2004
van Nes 2005
-
- Nes JG, Nortier WR, Velde CJ. The 4th large meta‐analysis of all trials of the treatment of operable breast cancer: increased survival after a longer follow‐up][Article in Dutch] . Nederlands Tijdschrift voor Geneeskunde 2005;149(26):1978‐80. - PubMed
Winter‐Roach 2009
World Health Organization [Computer program]
-
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems [World Health Organization classification of disease]. Version 10th Revision Version. WHO, 2007.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

