Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women
- PMID: 21975756
- PMCID: PMC6532702
- DOI: 10.1002/14651858.CD006689.pub2
Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women
Update in
-
Intermittent preventive treatment regimens for malaria in HIV-positive pregnant women.Cochrane Database Syst Rev. 2024 Sep 26;9(9):CD006689. doi: 10.1002/14651858.CD006689.pub3. Cochrane Database Syst Rev. 2024. PMID: 39324693 Free PMC article.
Abstract
Background: Intermittent preventive treatment is recommended for pregnant women living in malaria endemic countries due to benefits for both mother and baby. However, the impact may not be the same in HIV-positive pregnant women, as HIV infection impairs a woman's immunity.
Objectives: To compare intermittent preventive treatment regimens for malaria in HIV-positive pregnant women living in malaria-endemic areas.
Search strategy: In June 2011, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE; EMBASE; LILACS, the metaRegister of Controlled Trials (mRCT), reference lists and conference abstracts. We also contacted researchers and organizations for information on relevant trials.
Selection criteria: Randomized controlled trials comparing different intermittent preventive treatment regimens for preventing malaria in HIV-positive pregnant women in malaria-endemic areas.
Data collection and analysis: Two authors extracted data and assessed risk bias. Dichotomous variables were combined using risk ratios (RR) and mean differences (MD) for continuous outcomes, both with 95% confidence intervals (CI).
Main results: Two randomized trials with 722 HIV-positive pregnant women were included, comparing monthly regimens of sulfadoxine-pyrimethamine (SP) to the standard 2-dose regimen in the second and third trimesters. There were no statistically significant differences between monthly SP and 2-dose SP in rates of maternal anaemia, low birth weight, and neonatal mortality. In primigravidae and secondigravidiae, the monthly regimen was associated with less placental parasitaemia (RR 0.38, 95% CI 0.21 to 0.70, two trials) and less peripheral parasitaemia (RR 0.25, 95% CI 0.14 to 0.43, two trials), but no effect was demonstrated in multigravid women. Babies born to primigravidae and secundigravida women on monthly SP had a higher mean birth weight (weighted mean difference (WMD) 130 g; 95% CI 120 g to 150 g, two trials) than babies born to mothers on 2-dose SP. Multigravidae women treated with monthly SP had significant higher haemoglobin level than those treated with treated 2 dose SP (WMD 0.21 g/dL, 95% CI 0.15 g/dL to 0.27 g/dL, one trial). There were no trials that assessed other treatment regimens for intermittent preventive treatment in HIV-positive pregnant women.
Authors' conclusions: Three or more doses of SP is superior to the standard two doses in HIV-positive pregnant women. However, since SP cannot be administered concurrently with co-trimoxazole - a drug often recommended for infection prophylaxis in HIV-positive pregnant women, new drugs and research is needed to address needs of HIV-positive pregnant women.
Conflict of interest statement
None known for both authors.
Figures
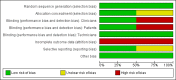

















References
References to studies included in this review
Filler 2006 {published data only}
-
- Filler S, Kazembe P, Thigpen M, Macheso A, Parise M, Newman R, et al. Randomized trial of 2‐dose versus monthly sulfadoxine‐pyrimethamine intermittent preventive treatment for malaria in HIV‐positive and HIV‐negative pregnant women in Malawi. Journal of Infectious Diseases 2006;194(3):286‐93. - PubMed
Hamer 2007 {published data only}
-
- Hamer D, Mwanakasale V, MacLeod W, Chalwe V, Mukwamataba D, Champo D, et al. Two‐dose versus monthly intermittent preventive treatment of malaria with sulfadoxine‐pyrimethamine in HIV‐seropositive pregnant Zambian women. Journal of Infectious Diseases 2007;196(11):1585‐94. - PubMed
References to studies excluded from this review
Parise 1998 {published data only}
-
- Parise M, Ayisi J, Nahlen B, Schultz L, Roberts J, Misore A, et al. Efficacy of sulfadoxine‐pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene 1998;59(5):813‐22. - PubMed
References to ongoing studies
Korukiiko {unpublished data only}
-
- "Influence of HIV infection on the effectiveness of malaria prevention during pregnancy, with emphasis on the effect of chloroquine on HIV viral load among pregnant women in Uganda". Ongoing study Not clear.
Macarthur {unpublished data only}
-
- "Efficacy of intermittent sulfadoxine‐pyrimethamine and sulfadoxine‐pyrimethamine + artesunate treatment in the prevention of malaria in pregnancy in an area with chloroquine‐resistant plasmodium falciparum". Ongoing study January 2003.
Manyando {unpublished data only}
-
- Establishing Effectiveness of Daily Co‐trimoxazole Prophylaxis For Prevention of Malaria in Pregnancy. Ongoing study April 2010.
Additional references
Abu‐Raddad 2006
-
- Abu‐Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub‐Saharan Africa. Science 2006;314(5805):1603‐6. - PubMed
Bijl 2000
-
- Bijl HM, Kager J, Koetsier DW, Werf TS. Chloroquine‐ and sulfadoxine‐pyrimethamine‐resistant Falciparum malaria in vivo ‐ a pilot study in rural Zambia. Tropical Medicine and International Health 2000;5(10):692‐5. - PubMed
Brahmbhatt 2003
-
- Brahmbhatt H, Kigozi G, Wabwire‐Mangen F, Serwadda D, Sewankambo N, Lutalo T, et al. The effects of placental malaria on mother‐to‐child HIV transmission in Rakai, Uganda. AIDS 2003;17(17):2539‐41. - PubMed
Chalwe 2009
Chico 2008
Cot 2003
-
- Cot M, Deloron P. Malaria prevention strategies. British Medical Bulletin 2003;67:137‐48. - PubMed
Dabis 2002
-
- Dabis F, Ekpini ER. HIV‐1/AIDS and maternal and child health in Africa. Lancet 2002;359(9323):2097‐104. - PubMed
French 2001
-
- French N, Nakiyingi J, Lugada E, Watera C, Whitworth JA, Gilks CF. Increasing rates of malarial fever with deteriorating immune status in HIV‐1‐infected Ugandan adults. AIDS 2001;15(7):899‐906. - PubMed
Froebel 2004
-
- Froebel K, Howard W, Schafer JR, Howie F, Whitworth J, Kaleebu P, et al. Activation by malaria antigens renders mononuclear cells susceptible to HIV infection and re‐activates replication of endogenous HIV in cells from HIV‐infected adults. Parasite Immunology 2004;26(5):213‐7. - PubMed
Garner 2006
-
- Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database of Systematic Reviews 2006, Issue 4. [Art. No.: CD000169. DOI: 10.1002/14651858.CD000169.pub2.] - PubMed
Higgins 2011
-
- Higgins JPT, Deeks JJ, Altman DG. [Chapter 16: Special topics in statistics]. In: Higgins JPT, Deeks JJ, Altman DG editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). updated March 2011, 2011.
Kapito‐Tembo 2011
-
- Kapito‐Tembo A, Meshnick SR, Hensbroek MB, Phiri K, Fitzgerald M, Mwapasa V. Marked reduction in prevalence of malaria parasitemia and anemia in HIV‐infected pregnant women taking cotrimoxazole with or without sulfadoxine‐pyrimethamine intermittent preventive therapy during pregnancy in Malawi. Journal of Infectious Diseases 2011;203(4):464‐72. - PMC - PubMed
Korenromp 2005
-
- Korenromp E, for the RBM Monitoring and Evaluation Reference Group & MERG Task Force on Malaria Morbidity. Malaria incidence estimates at country level for the year 2004 ‐ Proposed estimates and draft report. www.who.int/malaria/docs/incidence_estimations2.pdf 2005 (accessed on 22 Septermber 2006).
Korenromp 2005a
Kublin 2005
-
- Kublin JG, Patnaik P, Jere CS, Miller WC, Hoffman IF, Chimbiya N, et al. Effects of plasmodium falciparum malaria on concentration of HIV‐1‐RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet 2005;365(9455):233‐40. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies.. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011.
Malamba 2007
Malenga 2009
-
- Malenga G, Wirima J, Kazembe P, Nyasulu Y, Mbvundula M, Nyirenda C, et al. Developing national treatment policy for falciparum malaria in Africa: Malawi experience. Transactions of the Royal Society of Tropical Medicine and Hygiene 2009;103(Suppl 1):S15‐8. - PubMed
Msamanga 2009
Mulenga 2006
Parise 1998
-
- Parise ME, Ayisi JG, Nahlen BL, Schultz LJ, Roberts JM, Misore A, et al. Efficacy of sulfadoxine‐pyrimethamine for prevention of placental malaria in an area of Kenya with a high prevalence of malaria and human immunodeficiency virus infection. American Journal of Tropical Medicine and Hygiene 1998;59(5):813‐22. - PubMed
Perrault 2009
Review Manager 5.0 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Steketee 1996
-
- Steketee RW, Wirima JJ, Bloland PB, Chilima B, Mermin JH, Chitsulo L, et al. Impairment of a pregnant woman's acquired ability to limit plasmodium falciparum by infection with human immunodeficiency virus type‐1. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:42‐9. - PubMed
Tagbor 2006
-
- Tagbor H, Bruce J, Browne E, Randal A, Greenwood B, Chandramohan D. Efficacy, safety, and tolerability of amodiaquine plus sulphadoxine‐pyrimethamine used alone or in combination for malaria treatment in pregnancy: a randomised trial. Lancet 2006;368(9544):1349‐56. - PubMed
ter Kuile 2004
-
- ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, Eijk AM, et al. The burden of co‐infection with the human immunodeficiency virus type 1 and malaria in pregnant women in sub‐saharan Africa. American Journal of Tropical Medicine and Hygiene 2004;71(Suppl 2):41‐5. - PubMed
UNAIDS 2010
-
- United Nations Programme on HIV/AIDS. Epidemic update. UNAIDS Report on the global AIDS epidemic 2010. UNAIDS, 2010:16‐61.
Van Geertruyden 2006
-
- Geertruyden JP, Mulenga M, Mwananyanda L, Chalwe V, Moerman F, Chilengi R, et al. HIV‐1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. Journal of Infectious Diseases 2006;194(7):917‐25. - PubMed
Whitworth 2000
-
- Whitworth J, Morgan D, Quigley M, Smith A, Mayanja B, Eotu H, et al. Effect of HIV‐1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet 2000;356(9235):1051‐6. - PubMed
WHO 2004
-
- World Health Organization, Regional Office for Africa. A strategic framework for malaria prevention and control during pregnancy in the African region [AFR/MAL/04/01]. Brazzaville: World Health Organization, 2004.
WHO 2010
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Geneva: World Health Organization, 2010. - PubMed
WHO 2010a
-
- WHO/UNAIDS/UNICEF. Towards Universal Access: Scaling up priority HIV/AIDS interventions in the health sector. Geneva: WHO, 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

