Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia (ALL) in first complete remission
- PMID: 21975786
- PMCID: PMC7386902
- DOI: 10.1002/14651858.CD008818.pub2
Allogeneic hematopoietic cell transplantation for adult acute lymphoblastic leukemia (ALL) in first complete remission
Abstract
Background: Consolidation chemotherapy, autologous hematopoietic cell transplantation (HCT) and allogeneic HCT represent potential treatment alternatives for post-remission therapy in adult acute lymphoblastic leukemia (ALL), but there is genuine uncertainty regarding the optimal approach.
Objectives: To assess the effect of matched sibling donor vs. no donor status for adults with ALL in first complete remission (CR1).
Search strategy: We performed a search of CENTRAL, MEDLINE and EMBASE electronic databases in September 2010 along with handsearching of literature cited in relevant primary articles, search of abstracts from American Society of Hematology and American Society of Clinical Oncology meetings, as well as consultation with content experts in the field.
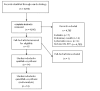
Selection criteria: Review was performed by two authors, and Inclusion criteria included the following: controlled trials with donor vs. no donor comparison with assignment by genetic randomizationin adults with ALL in CR1.
Data collection and analysis: We extracted data on benefits (overall survival, progression-free survival) and harms (treatment-related mortality, relapse) of compared treatments. Adverse events were considered, but analysis of individual adverse events was not possible from the reported literature. We pooled summary results from each study using a random-effects model. We assessed heterogeneity. We performed subgroup analyses for disease risk categories. We performed sensitivity analyses according to methodological quality.
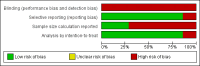
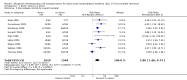
Main results: A total of 14 relevant trials were identified, consisting of a total of 3157 patients. There was a statistically significant overall survival advantage in favor of the donor versus no donor group (HR 0.86; 95% CI 0.77 to 0.97; P = 0.01), as well as significant improvement in disease-free survival in the donor group(HR 0.82; 95% CI 0.72 to 0.94; P = 0.004). Those in the donor group had significant reduction in primary disease relapse(RR 0.53; 95% CI 0.37 to 0.76; P = 0.0004) and significant increase in non-relapse mortality(RR 2.8; 95% CI 1.66 to 4.73; P = 0.001). Significant heterogeneity was detected in analysis of relapse (Chi(2) 40.51, df = 6, P < 0.00001; I(2) = 85%). In regard to methodologic quality, the majority of included studies were free of selective reporting, and performed analyses according to intention to treat. Conversely, few reported sample size calculation that informed the study design. While blinding was considered as an important domain of methodological quality, none of the studies reported on whether any of the study personnel were blinded (e.g. subjects, personnel, outcome assessors, data analysts etc). Therefore, we did not consider blinding further in the analysis of methodological quality in this review.
Authors' conclusions: The results of this systematic review and meta-analysis support matched sibling donor allogeneic hematopoietic cell transplantation as the optimal post-remission therapy in ALL patients aged 15 years or over. This therapy offers superior overall survival and disease-free survival, and significantly reduces the risk of disease relapse, but does impose an increased risk of non-relapse mortality. Importantly these data are based on adult ALL treated with largely total body irradiation-based myeloablative conditioning and sibling donor transplantation and, therefore, cannot be generalized to pediatric ALL, alternative donors including HLA (human leukocyte antigen) mismatched or unrelated donors, or reduced toxicity or non-myeloablative conditioning regimens.
Conflict of interest statement
Dr. Kharfan‐Dabaja has received payment for lectures from Genzyme and Bristol Myers Squibb. He has also received consultancy fees from Vical. These financial activities are not relevant to the submitted work. All other authors report no conflict of interest.
Figures
















Update of
References
References to studies included in this review
Attal 1995 {published data only}
-
- Attal M, Blaise D, Marit G, Payen C, Michallet M, Vernant JP, et al. Consolidation treatment of adult acute lymphoblastic leukemia: a prospective, randomized trial comparing allogeneic versus autologous bone marrow transplantation and testing the impact of recombinant interleukin‐2 after autologous bone marrow transplantation. BGMT Group. Blood 1995;86(4):1619‐28. - PubMed
Bernasconi 1992 {published data only}
-
- Bernasconi C, Lazzarino M, Morra E, Alessandrino EP, Pagnucco G, Resegotti L, et al. Early intensification followed by allo‐BMT or auto‐BMT or a second intensification in adult ALL: a randomized multicenter study. Leukemia 1992;6:204‐8. - PubMed
Cornelissen 2009 {published data only}
-
- Cornelissen JJ, Holt B, Verhoef GE, van't Veer MB, Oers MH, Schouten HC, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no‐donor comparison. Blood 2009;113(6):1375‐82. - PubMed
De Witte 1994 {published data only}
-
- Witte T, Awwad B, Boezeman J, Schattenberg A, Muus P, Raemaekers J, et al. Role of allogenic bone marrow transplantation in adolescent or adult patients with acute lymphoblastic leukaemia or lymphoblastic lymphoma in first remission. Bone Marrow Transplant 1994;14(5):767‐74. - PubMed
Fielding 2009 {published data only}
-
- Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome‐positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre‐imatinib era: results from the International ALL Trial MRC UKALLXI /ECOG2993. Blood 2009;113(19):4489‐96. - PMC - PubMed
Goldstone 2008 {published data only}
-
- Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard‐risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood 2008;111(4):1827‐33. [PUBMED: 18048644] - PubMed
Hunault 2004 {published data only}
-
- Hunault M, Harousseau JL, Delain M, Truchan‐Graczyk M, Cahn JY, Witz F, et al. Better outcome of adult acute lymphoblastic leukemia after early genoidentical allogeneic bone marrow transplantation (BMT) than after late high‐dose therapy and autologous BMT: a GOELAMS trial. Blood 2004;104(10):3028‐37. [PUBMED: 15256423] - PubMed
Ifrah 1999 {published data only}
-
- Ifrah N, Witz F, Jouet JP, François S, Lamy T, Linassier C, et al. Intensive short term therapy with granulocyte‐macrophage‐colony stimulating factor support, similar to therapy for acute myeloblastic leukemia, does not improve overall results for adults with acute lymphoblastic leukemia. GOELAMS Group. Cancer 1999;86((8)):1496‐505. - PubMed
Labar 2004 {published data only}
-
- Labar B, Suciu S, Zittoun R, Muus P, Marie JP, Fillet G, et al. Allogeneic stem cell transplantation in acute lymphoblastic leukemia and non‐Hodgkin's lymphoma for patients < or = 50 years old in first complete remission: results of the EORTC ALL‐3 trial. Haematologica 2004;89(7):809‐17. - PubMed
Ribera 2005 {published data only}
-
- Ribera JM, Oriol A, Bethencourt C, Parody R, Hernandez‐Rivas JM, Moreno MJ, et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post‐remission treatment for adult patients with high‐risk acute lymphoblastic leukemia. Results of the PETHEMA ALL‐93 trial. Haematologica 2005;90(10):1346‐56. - PubMed
Sebban 1994 {published data only}
-
- Sebban C, Lepage E, Vernant JP, Gluckman E, Attal M, Reiffers J, et al. Allogeneic bone marrow transplantation in adult acute lymphoblastic leukemia in first complete remission: a comparative study. French Group of Therapy of Adult Acute Lymphoblastic Leukemia. Journal of Clinical Oncology 1994;12(12):2580‐7. - PubMed
Takeuchi 2002 {published data only}
-
- Takeuchi J, Kyo T, Naito K, Sao H, Takahashi M, Miyawaki S, et al. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG‐ALL93 study. Leukemia 2002;16(7):1259‐66. - PubMed
Thomas 2004 {published data only}
-
- Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA‐94 trial. Journal of Clinical Oncology 2004;22(20):4075‐86. - PubMed
Ueda 1998 {published data only}
-
- Ueda T, Miyawaki S, Asou N, Kuraishi Y, Hiraoka A, Kuriyama K, et al. Response‐oriented individualized induction therapy with six drugs followed by four courses of intensive consolidation, 1 year maintenance and intensification therapy: the ALL90 study of the Japan Adult Leukemia Study Group. International Journal of Hematology 1998;68(3):279‐89. - PubMed
Additional references
Als‐Nielsen 2004
-
- Als‐Nielsen B, Gluud LL, Gluud C. Methodological quality and treatment effects in randomized trials: a review of six empirical studies. 12th Cochrane Colloquium. 2004.
Annino 2002
-
- Annino L, Vegna ML, Camera A, Specchia G, Visani G, Fioritoni G, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long‐term follow‐up of the GIMEMA ALL 0288 randomized study. Blood 2002;99:863‐71. - PubMed
Bassan 2001
-
- Bassan R, Pogliani E, Casula P, Rossi G, Fabris P, Morandi S, et al. Risk‐oriented post‐remission strategies in adult acute lymphoblastic leukemia: prospective confirmation of anthracycline activity in standard‐risk class and role of hematopoietic stem cell transplants in high‐risk groups. Hematology Journal 2001;2:117‐26. - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;315:1088‐101. - PubMed
Boutron 2005
-
- Boutron I, Estellat C, Ravaud P. A review of blinding in randomized controlled trials found results inconsistent and questionable. Journal of Clinical Epidemiology 2005;58:1220‐6. - PubMed
Chan 2005
Deeks 2008
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, 2008.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Dombret 2002a
-
- Dombret H, Gabert J, Boiron JM, Rigal‐Huguet F, Blaise D, Thomas X, et al. Outcome of treatment in adults with Philadelphia chromosome‐positive acute lymphoblastic leukemia: results of the prospective multicenter LALA‐94 trial. Blood 2002;100(7):2357‐66. - PubMed
Egger 1997
Hallbrook 2002
-
- Hallbrook H, Simonsson B, Ahlgren T, Bjorkholm M, Carneskog J, Grimfors G, et al. High‐dose cytarabine in upfront therapy for adult patients with acute lymphoblastic leukaemia. British Journal of Haematology 2002;118:748‐54. - PubMed
Haynes 2005
Higgins 2002
-
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. - PubMed
Higgins 2003
Higgins 2008
-
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, 2008.
Holowiecki 2008
-
- Holowiecki J, Krawczyk‐Kulis M, Giebel S, Jagoda K, Stella‐Holowiecka B, Piatkowska‐Jakubas B, et al. Status of minimal residual disease after induction predicts outcome in both standard and high‐risk Ph‐negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4‐2002 MRD Study. British Journal of Haematology 2008;142(2):227‐37. - PubMed
Jüni 2001
Kantarjian 2004
-
- Kantarjian H, Thomas D, O'Brien S, Cortes J, Giles F, Jeha S, et al. Long‐term follow‐up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper‐CVAD), a dose‐intensive regimen, in adult acute lymphocytic leukemia. Cancer 2004;101:2788‐801. - PubMed
Larson 1998
-
- Larson RA, Dodge RK, Linker CA, Stone RM, Powell BL, Lee EJ, et al. A randomized controlled trial of filgrastim during remission induction and consolidation chemotherapy for adults with acute lymphoblastic leukemia: CALGB study 9111. Blood 1998;92:1556‐64. - PubMed
Linker 2002
-
- Linker C, Damon L, Ries C, Navarro W. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia. Journal of Clinical Oncology 2002;20:2464‐71. - PubMed
Moher 1999
-
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;354:1896‐900. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Peters 2006
-
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta‐analysis. JAMA 2006;95:676‐80. - PubMed
Ram 2010
-
- Ram R, Gafter‐Gvili A, Vidal L, Paul M, Ben‐Bassat I, Shpilberg O, et al. Management of adult patients with acute lymphoblastic leukemia in first complete remission: systematic review and meta‐analysis. Cancer 2010;116:3447‐57. - PubMed
Schulz 2002
-
- Schulz KF, Grimes DA. Unequal group sizes in randomised trials: guarding against guessing. Lancet 2002;359:966‐70. - PubMed
Stanulla 2009
-
- Stanulla M, Schrappe M. Treatment of childhood acute lymphoblastic leukemia. Seminars in Hematology 2009;46:52‐63. - PubMed
Sterne 2001
-
- Sterne JAC, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54:1046‐55. - PubMed
Sterne 2008
-
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. John Wiley & Sons, Ltd, 2008.
Thiebaut 2000
-
- Thiebaut A, Vernant JP, Degos L, Huguet FR, Reiffers J, Sebban C, et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow‐up report of the French protocol LALA‐87. Hematology Oncology Clinics of North America 2000;14:1353‐66. - PubMed
Wheatley 2004
-
- Wheatley K, Gray R. Commentary: Mendelian randomization? An update on its use to evaluate allogeneic stem cell transplantation in leukaemia. International Journal of Epidemiology 2004;33:15‐7. - PubMed
Yanada 2006
-
- Yanada M, Matsuo K, Suzuki T, Naoe T. Allogeneic hematopoietic stem cell transplantation as part of post‐remission therapy improves survival for adult patients with high‐risk acute lymphoblastic leukemia: a meta‐analysis. Cancer 2006;106:2657‐63. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

