Hindered submicron mobility and long-term storage of presynaptic dense-core granules revealed by single-particle tracking
- PMID: 21976424
- PMCID: PMC3512567
- DOI: 10.1002/dneu.20984
Hindered submicron mobility and long-term storage of presynaptic dense-core granules revealed by single-particle tracking
Abstract
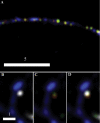
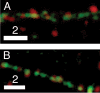
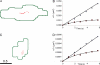
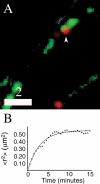
Dense-core granules (DCGs) are organelles found in neuroendocrine cells and neurons that house, transport, and release a number of important peptides and proteins. In neurons, DCG cargo can include the secreted neuromodulatory proteins tissue plasminogen activator (tPA) and/or brain-derived neurotrophic factor (BDNF), which play a key role in modulating synaptic efficacy in the hippocampus. This function has spurred interest in DCGs that localize to synaptic contacts between hippocampal neurons, and several studies recently have established that DCGs localize to, and undergo regulated exocytosis from, postsynaptic sites. To complement this work, we have studied presynaptically localized DCGs in hippocampal neurons, which are much more poorly understood than their postsynaptic analogs. Moreover, to enhance relevance, we visualized DCGs via fluorescence labeling of exogenous and endogenous tPA and BDNF. Using single-particle tracking, we determined trajectories of more than 150 presynaptically localized DCGs. These trajectories reveal that mobility of DCGs in presynaptic boutons is highly hindered and that storage is long-lived. We also computed mean-squared displacement curves, which can be used to elucidate mechanisms of transport. Over shorter time windows, most curves are linear, demonstrating that DCG transport in boutons is driven predominantly by diffusion. The remaining curves plateau with time, consistent with motion constrained by a submicron-sized corral. These results have relevance to recent models of presynaptic organization and to recent hypotheses about DCG cargo function. The results also provide estimates for transit times to the presynaptic plasma membrane that are consistent with measured times for onset of neurotrophin release from synaptically localized DCGs.
Copyright © 2011 Wiley Periodicals, Inc.
Figures

References
-
- Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renstrom E, Rorsman P. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. - PubMed
-
- Benchenane K, Lopez-Atalaya JP, Fernandez-Monreal M, Touzani O, Vivien D. Equivocal roles of tissue-type plasminogen activator in stroke-induced injury. Trends Neurosci. 2004;27:155–160. - PubMed
-
- Benson DL, Salton SR. Expression and polarization of VGF in developing hippocampal neurons. Brain Res Dev Brain Res. 1996;96:219–228. - PubMed
-
- Berezhkovskii AM, Grigoriev IV, Makhnovskii YA, Zitserman VY. Kinetics of escape through a small hole. Journal of Chemical Physics. 2002;116:9574–9577.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

