Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia
- PMID: 21976486
- PMCID: PMC3198321
- DOI: 10.1073/pnas.1114026108
Priming of hypoxia-inducible factor by neuronal nitric oxide synthase is essential for adaptive responses to severe anemia
Abstract
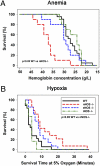
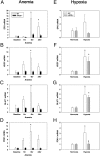
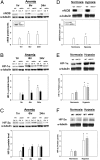
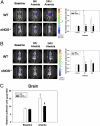
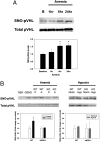
Cells sense and respond to changes in oxygen concentration through gene regulatory processes that are fundamental to survival. Surprisingly, little is known about how anemia affects hypoxia signaling. Because nitric oxide synthases (NOSs) figure prominently in the cellular responses to acute hypoxia, we defined the effects of NOS deficiency in acute anemia. In contrast to endothelial NOS or inducible NOS deficiency, neuronal NOS (nNOS)(-/-) mice demonstrated increased mortality during anemia. Unlike wild-type (WT) animals, anemia did not increase cardiac output (CO) or reduce systemic vascular resistance (SVR) in nNOS(-/-) mice. At the cellular level, anemia increased expression of HIF-1α protein and HIF-responsive mRNA levels (EPO, VEGF, GLUT1, PDK1) in the brain of WT, but not nNOS(-/-) mice, despite comparable reductions in tissue PO(2). Paradoxically, nNOS(-/-) mice survived longer during hypoxia, retained the ability to regulate CO and SVR, and increased brain HIF-α protein levels and HIF-responsive mRNA transcripts. Real-time imaging of transgenic animals expressing a reporter HIF-α(ODD)-luciferase chimeric protein confirmed that nNOS was essential for anemia-mediated increases in HIF-α protein stability in vivo. S-nitrosylation effects the functional interaction between HIF and pVHL. We found that anemia led to nNOS-dependent S-nitrosylation of pVHL in vivo and, of interest, led to decreased expression of GSNO reductase. These findings identify nNOS effects on the HIF/pVHL signaling pathway as critically important in the physiological responses to anemia in vivo and provide essential mechanistic insight into the differences between anemia and hypoxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Carson JL, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. - PubMed
-
- Karkouti K, Wijeysundera DN, Beattie WS. Reducing Bleeding in Cardiac Surgery (RBC) Investigators (2008) Risk associated with preoperative anemia in cardiac surgery: A multicenter cohort study. Circulation. 117:478–484. - PubMed
-
- Anand I, et al. Anemia and its relationship to clinical outcome in heart failure. Circulation. 2004;110:149–154. - PubMed
-
- Pfeffer MA, et al. TREAT Investigators (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 361:2019–2032. - PubMed
-
- Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: A systematic review of the literature. Am J Med. 2004;116(Suppl 7A):11S–26S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

