Regulation of neuronal M-channel gating in an isoform-specific manner: functional interplay between calmodulin and syntaxin 1A
- PMID: 21976501
- PMCID: PMC6623657
- DOI: 10.1523/JNEUROSCI.2666-11.2011
Regulation of neuronal M-channel gating in an isoform-specific manner: functional interplay between calmodulin and syntaxin 1A
Abstract
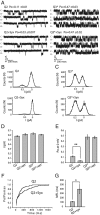
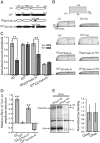
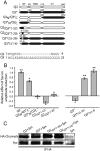
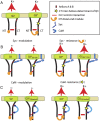
Whereas neuronal M-type K(+) channels composed of KCNQ2 and KCNQ3 subunits regulate firing properties of neurons, presynaptic KCNQ2 subunits were demonstrated to regulate neurotransmitter release by directly influencing presynaptic function. Two interaction partners of M-channels, syntaxin 1A and calmodulin, are known to act presynaptically, syntaxin serving as a major protein component of the membrane fusion machinery and calmodulin serving as regulator of several processes related to neurotransmitter release. Notably, both partners specifically modulate KCNQ2 but not KCNQ3 subunits, suggesting selective presynaptic targeting to directly regulate exocytosis without interference in neuronal firing properties. Here, having first demonstrated in Xenopus oocytes, using analysis of single-channel biophysics, that both modulators downregulate the open probability of KCNQ2 but not KCNQ3 homomers, we sought to resolve the channel structural determinants that confer the isoform-specific gating downregulation and to get insights into the molecular events underlying this mechanism. We show, using optical, biochemical, electrophysiological, and molecular biology analyses, the existence of constitutive interactions between the N and C termini in homomeric KCNQ2 and KCNQ3 channels in living cells. Furthermore, rearrangement in the relative orientation of the KCNQ2 termini that accompanies reduction in single-channel open probability is induced by both regulators, strongly suggesting that closer N-C termini proximity underlies gating downregulation. Different structural determinants, identified at the N and C termini of KCNQ3, prevent the effects by syntaxin 1A and calmodulin, respectively. Moreover, we show that the syntaxin 1A and calmodulin effects can be additive or blocked at different concentration ranges of calmodulin, bearing physiological significance with regard to presynaptic exocytosis.
Figures





References
-
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. - PubMed
-
- Bofill-Cardona E, Kudlacek O, Yang Q, Ahorn H, Freissmuth M, Nanoff C. Binding of calmodulin to the D2-dopamine receptor reduces receptor signaling by arresting the G protein activation switch. J Biol Chem. 2000;275:32672–32680. - PubMed
-
- Bright JN, Sansom MSP. The flexing/twirling helix: exploring the flexibility about molecular hinges formed by praline and glycine motifs in transmembrane helices. J Phys Chem B. 2003;107:627–636.
-
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
