Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry
- PMID: 21976507
- PMCID: PMC3207280
- DOI: 10.1523/JNEUROSCI.3157-11.2011
Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry
Abstract
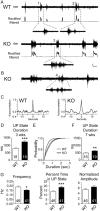
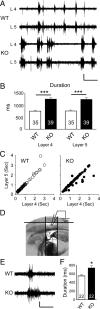
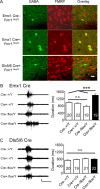
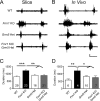
Despite the pronounced neurological deficits associated with mental retardation and autism, the degree to which neocortical circuit function is altered remains unknown. Here, we study changes in neocortical network function in the form of persistent activity states in the mouse model of fragile X syndrome--the Fmr1 knock-out (KO). Persistent activity states, or UP states, in the neocortex underlie the slow oscillation which occurs predominantly during slow-wave sleep, but may also play a role during awake states. We show that spontaneously occurring UP states in the primary somatosensory cortex are 38-67% longer in Fmr1 KO slices. In vivo, UP states reoccur with a clear rhythmic component consistent with that of the slow oscillation and are similarly longer in the Fmr1 KO. Changes in neocortical excitatory circuitry likely play the major role in this alteration as supported by three findings: (1) longer UP states occur in slices of isolated neocortex, (2) pharmacologically isolated excitatory circuits in Fmr1 KO neocortical slices display prolonged bursting states, and (3) selective deletion of Fmr1 in cortical excitatory neurons is sufficient to cause prolonged UP states whereas deletion in inhibitory neurons has no effect. Excess signaling mediated by the group 1 glutamate metabotropic receptor, mGluR5, contributes to the longer UP states. Genetic reduction or pharmacological blockade of mGluR5 rescues the prolonged UP state phenotype. Our results reveal an alteration in network function in a mouse model of intellectual disability and autism which may impact both slow-wave sleep and information processing during waking states.
Figures




Similar articles
-
Selective Disruption of Metabotropic Glutamate Receptor 5-Homer Interactions Mimics Phenotypes of Fragile X Syndrome in Mice.J Neurosci. 2016 Feb 17;36(7):2131-47. doi: 10.1523/JNEUROSCI.2921-15.2016. J Neurosci. 2016. PMID: 26888925 Free PMC article.
-
Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model for fragile X syndrome.Behav Brain Res. 2011 Oct 1;223(2):310-21. doi: 10.1016/j.bbr.2011.04.049. Epub 2011 May 6. Behav Brain Res. 2011. PMID: 21571007 Free PMC article.
-
Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome.J Neurophysiol. 2008 Nov;100(5):2615-26. doi: 10.1152/jn.90752.2008. Epub 2008 Sep 10. J Neurophysiol. 2008. PMID: 18784272 Free PMC article.
-
GABA receptor subunit distribution and FMRP-mGluR5 signaling abnormalities in the cerebellum of subjects with schizophrenia, mood disorders, and autism.Schizophr Res. 2015 Sep;167(1-3):42-56. doi: 10.1016/j.schres.2014.10.010. Epub 2014 Nov 26. Schizophr Res. 2015. PMID: 25432637 Free PMC article. Review.
-
Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development.Psychopharmacology (Berl). 2014 Mar;231(6):1217-26. doi: 10.1007/s00213-013-3330-3. Psychopharmacology (Berl). 2014. PMID: 24232444 Review.
Cited by
-
Cell-Type Specific Channelopathies in the Prefrontal Cortex of the fmr1-/y Mouse Model of Fragile X Syndrome.eNeuro. 2015 Nov 17;2(6):ENEURO.0114-15.2015. doi: 10.1523/ENEURO.0114-15.2015. eCollection 2015 Nov-Dec. eNeuro. 2015. PMID: 26601124 Free PMC article.
-
mTORC1 Is a Local, Postsynaptic Voltage Sensor Regulated by Positive and Negative Feedback Pathways.Front Cell Neurosci. 2017 May 30;11:152. doi: 10.3389/fncel.2017.00152. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28611595 Free PMC article.
-
Cerebellar Dysfunction in Autism Spectrum Disorders: Deriving Mechanistic Insights from an Internal Model Framework.Neuroscience. 2021 May 10;462:274-287. doi: 10.1016/j.neuroscience.2020.11.012. Epub 2020 Nov 28. Neuroscience. 2021. PMID: 33253824 Free PMC article. Review.
-
Neural Hyperexcitability in Autism Spectrum Disorders.Brain Sci. 2017 Oct 13;7(10):129. doi: 10.3390/brainsci7100129. Brain Sci. 2017. PMID: 29027913 Free PMC article. Review.
-
Glutamate induces the elongation of early dendritic protrusions via mGluRs in wild type mice, but not in fragile X mice.PLoS One. 2012;7(2):e32446. doi: 10.1371/journal.pone.0032446. Epub 2012 Feb 27. PLoS One. 2012. PMID: 22384253 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Amzica F, Steriade M. Cellular substrates and laminar profile of sleep K-complex. Neuroscience. 1998;82:671–686. - PubMed
-
- Bagni C, Greenough WT. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci. 2005;6:376–387. - PubMed
-
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
