Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation
- PMID: 21976515
- PMCID: PMC6623673
- DOI: 10.1523/JNEUROSCI.2890-11.2011
Alterations of molecular and behavioral responses to cocaine by selective inhibition of Elk-1 phosphorylation
Abstract
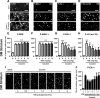
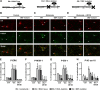
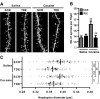
Activation of the extracellular signal-regulated kinase (ERK) signaling pathway in the striatum is crucial for molecular adaptations and long-term behavioral alterations induced by cocaine. In response to cocaine, ERK controls the phosphorylation levels of both mitogen and stress-activated protein kinase 1 (MSK-1), a nuclear kinase involved in histone H3 (Ser10) and cAMP response element binding protein phosphorylation, and Elk-1, a transcription factor involved in serum response element (SRE)-driven gene regulations. We recently characterized the phenotype of msk-1 knock-out mice in response to cocaine. Herein, we wanted to address the role of Elk-1 phosphorylation in cocaine-induced molecular, morphological, and behavioral responses. We used a cell-penetrating peptide, named TAT-DEF-Elk-1 (TDE), which corresponds to the DEF docking domain of Elk-1 toward ERK and inhibits Elk-1 phosphorylation induced by ERKs without modifying ERK or MSK-1 in vitro. The peptide was injected in vivo before cocaine administration in mice. Immunocytochemical, molecular, morphological, and behavioral studies were performed. The TDE inhibited Elk-1 and H3 (Ser10) phosphorylation induced by cocaine, sparing ERK and MSK-1 activation. Consequently, TDE altered cocaine-induced regulation of genes bearing SRE site(s) in their promoters, including c-fos, zif268, ΔFosB, and arc/arg3.1 (activity-regulated cytoskeleton-associated protein). In a chronic cocaine administration paradigm, TDE reversed cocaine-induced increase in dendritic spine density. Finally, the TDE delayed the establishment of cocaine-induced psychomotor sensitization and conditioned-place preference. We conclude that Elk-1 phosphorylation downstream from ERK is a key molecular event involved in long-term neuronal and behavioral adaptations to cocaine.
Figures





References
-
- Brami-Cherrier K, Valjent E, Hervé D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25:11444–11454. [Erratum (2006) 26:table of contents; Simon, Arthur J corrected to Arthur, Simon J] - PMC - PubMed
-
- Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108:1323–1335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
