Annexin A2 promotes glioma cell invasion and tumor progression
- PMID: 21976520
- PMCID: PMC3201988
- DOI: 10.1523/JNEUROSCI.3299-11.2011
Annexin A2 promotes glioma cell invasion and tumor progression
Abstract
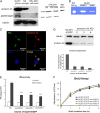
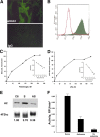
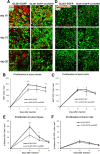
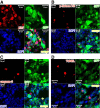
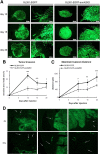
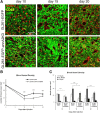
Gliomas are highly invasive, lethal brain tumors. Tumor-associated proteases play an important role in glioma progression. Annexin A2 is overexpressed in many cancers and correlates with increased plasmin activity on the tumor cell surface, which mediates degradation of extracellular matrix and promotes neoangiogenesis to facilitate tumor growth. In this study, we used two glioma cell lines, mouse GL261-EGFP and rat C6/LacZ, as well as stable clones transfected with an annexin A2 knockdown construct. We find that the annexin A2 knockdown decreased glioma cell migration in vitro and decreased membrane-bound plasmin activity. In vivo, we injected the glioma cells into the rodent brain and followed glioma progression. Knockdown of annexin A2 in glioma cells decreased tumor size and slowed tumor progression, as evidenced by decreased invasion, angiogenesis, and proliferation, as well as increased apoptosis in the tumor tissue of the annexin A2 knockdown group. Moreover, we report that the levels of expression of annexin A2 in human glioma samples correlate with their degree of malignancy. Together, our findings demonstrate that inhibition of annexin A2 expression in glioma cells could become a new target for glioma therapy.
Figures




Similar articles
-
Knockdown of annexin 2 decreases migration of human glioma cells in vitro.Neuropathol Appl Neurobiol. 2006 Jun;32(3):271-7. doi: 10.1111/j.1365-2990.2006.00720.x. Neuropathol Appl Neurobiol. 2006. PMID: 16640645
-
Annexin A2 regulates angiogenesis and invasion phenotypes of malignant glioma.Brain Tumor Pathol. 2015 Jul;32(3):184-94. doi: 10.1007/s10014-015-0216-6. Epub 2015 Feb 20. Brain Tumor Pathol. 2015. PMID: 25697644
-
Expression of annexin II in glioma cell lines and in brain tumor biopsies.J Neurooncol. 1998 May;38(1):11-8. doi: 10.1023/a:1005953000523. J Neurooncol. 1998. PMID: 9540053
-
S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors.Front Biosci. 2005 Jan 1;10:300-25. doi: 10.2741/1529. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574370 Review.
-
The role of annexin II in angiogenesis and tumor progression: a potential therapeutic target.Curr Pharm Des. 2007;13(35):3568-75. doi: 10.2174/138161207782794167. Curr Pharm Des. 2007. PMID: 18220793 Review.
Cited by
-
Annexin A2 in Inflammation and Host Defense.Cells. 2020 Jun 19;9(6):1499. doi: 10.3390/cells9061499. Cells. 2020. PMID: 32575495 Free PMC article. Review.
-
A novel DNA aptamer targeting lung cancer stem cells exerts a therapeutic effect by binding and neutralizing Annexin A2.Mol Ther Nucleic Acids. 2022 Jan 19;27:956-968. doi: 10.1016/j.omtn.2022.01.012. eCollection 2022 Mar 8. Mol Ther Nucleic Acids. 2022. PMID: 35211356 Free PMC article.
-
The Annexin a2 Promotes Development in Arthritis through Neovascularization by Amplification Hedgehog Pathway.PLoS One. 2016 Mar 10;11(3):e0150363. doi: 10.1371/journal.pone.0150363. eCollection 2016. PLoS One. 2016. PMID: 26963384 Free PMC article.
-
Annexin A2: regulating glioma's fate and a potential therapeutic target.Med Oncol. 2025 Jul 28;42(9):386. doi: 10.1007/s12032-025-02799-x. Med Oncol. 2025. PMID: 40720044
-
Clinical and prognostic role of annexin A2 in adamantinomatous craniopharyngioma.J Neurooncol. 2017 Jan;131(1):21-29. doi: 10.1007/s11060-016-2273-z. Epub 2016 Sep 17. J Neurooncol. 2017. PMID: 27640198
References
-
- Allegranza A, Girlando S, Arrigoni GL, Veronese S, Mauri FA, Gambacorta M, Pollo B, Dalla Palma P, Barbareschi M. Proliferating cell nuclear antigen expression in central nervous system neoplasms. Virchows Arch A Pathol Anat Histopathol. 1991;419:417–423. - PubMed
-
- Amberger VR, Hensel T, Ogata N, Schwab ME. Spreading and migration of human glioma and rat C6 cells on central nervous system myelin in vitro is correlated with tumor malignancy and involves a metalloproteolytic activity. Cancer Res. 1998;58:149–158. - PubMed
-
- Bao H, Jiang M, Zhu M, Sheng F, Ruan J, Ruan C. Overexpression of Annexin II affects the proliferation, apoptosis, invasion and production of proangiogenic factors in multiple myeloma. Int J Hematol. 2009;90:177–185. - PubMed
-
- Beckner ME, Chen X, An J, Day BW, Pollack IF. Proteomic characterization of harvested pseudopodia with differential gel electrophoresis and specific antibodies. Lab Invest. 2005;85:316–327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
