Heightened nicotinic regulation of auditory cortex during adolescence
- PMID: 21976522
- PMCID: PMC3210642
- DOI: 10.1523/JNEUROSCI.1705-11.2011
Heightened nicotinic regulation of auditory cortex during adolescence
Abstract
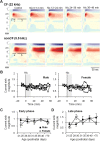
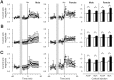
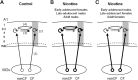
Adolescent smoking is associated with auditory-cognitive deficits and structural alterations to auditory thalamocortical systems, suggesting that higher auditory function is vulnerable to nicotine exposure during adolescence. Although nicotinic acetylcholine receptors (nAChRs) regulate thalamocortical processing in adults, it is not known whether they regulate processing at earlier ages since their expression pattern changes throughout postnatal development. Here we investigate nicotinic regulation of tone-evoked current source density (CSD) profiles in mouse primary auditory cortex from just after hearing onset until adulthood. At the youngest ages, systemic nicotine did not affect CSD profiles. However, beginning in early adolescence nicotine enhanced characteristic frequency (CF)-evoked responses in layers 2-4 by enhancing thalamocortical, early intracortical, and late intracortical response components. Nicotinic responsiveness developed rapidly and peaked over the course of adolescence, then declined thereafter. Generally, responsiveness in females developed more quickly, peaked earlier, and declined more abruptly and fully than in males. In contrast to the enhancement of CF-evoked responses, nicotine suppressed shorter-latency intracortical responses to spectrally distant (non-CF) stimuli while enhancing longer-latency responses. Intracortical infusion of nAChR antagonists showed that enhancement of CF-evoked intracortical processing involves α4β2*, but not α7, nAChRs, whereas both receptor subtypes regulate non-CF-evoked late intracortical responses. Notably, antagonist effects in females implied regulation by endogenous acetylcholine. Thus, nicotinic regulation of cortical processing varies with age and sex, with peak effects during adolescence that may contribute to the vulnerability of adolescents to smoking.
Figures

Similar articles
-
Nicotinic neuromodulation in auditory cortex requires MAPK activation in thalamocortical and intracortical circuits.J Neurophysiol. 2012 May;107(10):2782-93. doi: 10.1152/jn.01129.2011. Epub 2012 Feb 22. J Neurophysiol. 2012. PMID: 22357798 Free PMC article.
-
Nicotinic modulation of tone-evoked responses in auditory cortex reflects the strength of prior auditory learning.Neurobiol Learn Mem. 2008 Jul;90(1):138-46. doi: 10.1016/j.nlm.2008.02.006. Epub 2008 Apr 18. Neurobiol Learn Mem. 2008. PMID: 18378471 Free PMC article.
-
Systemic Nicotine Increases Gain and Narrows Receptive Fields in A1 via Integrated Cortical and Subcortical Actions.eNeuro. 2017 Jun 22;4(3):ENEURO.0192-17.2017. doi: 10.1523/ENEURO.0192-17.2017. eCollection 2017 May-Jun. eNeuro. 2017. PMID: 28660244 Free PMC article.
-
Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation.Ann N Y Acad Sci. 2014 Oct;1327(1):27-45. doi: 10.1111/nyas.12421. Epub 2014 Apr 14. Ann N Y Acad Sci. 2014. PMID: 24730978 Free PMC article. Review.
-
Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia.Psychopharmacology (Berl). 2004 Jun;174(1):54-64. doi: 10.1007/s00213-003-1750-1. Epub 2004 Feb 19. Psychopharmacology (Berl). 2004. PMID: 15205879 Review.
Cited by
-
Null mutations in EphB receptors decrease sharpness of frequency tuning in primary auditory cortex.PLoS One. 2011;6(10):e26192. doi: 10.1371/journal.pone.0026192. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022561 Free PMC article.
-
Nicotine and the adolescent brain.J Physiol. 2015 Aug 15;593(16):3397-412. doi: 10.1113/JP270492. Epub 2015 Jun 23. J Physiol. 2015. PMID: 26018031 Free PMC article. Review.
-
Spectral breadth and laminar distribution of thalamocortical inputs to A1.J Neurophysiol. 2016 Apr;115(4):2083-94. doi: 10.1152/jn.00887.2015. Epub 2016 Feb 17. J Neurophysiol. 2016. PMID: 26888102 Free PMC article.
-
Nicotinic neuromodulation in auditory cortex requires MAPK activation in thalamocortical and intracortical circuits.J Neurophysiol. 2012 May;107(10):2782-93. doi: 10.1152/jn.01129.2011. Epub 2012 Feb 22. J Neurophysiol. 2012. PMID: 22357798 Free PMC article.
-
Nicotinic regulation of experience-dependent plasticity in visual cortex.J Physiol Paris. 2016 Sep;110(1-2):29-36. doi: 10.1016/j.jphysparis.2016.11.003. Epub 2016 Nov 10. J Physiol Paris. 2016. PMID: 27840212 Free PMC article. Review.
References
-
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. - PubMed
-
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992;41:802–808. - PubMed
-
- Anderson LA, Christianson GB, Linden JF. Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res. 2009;1252:130–142. - PubMed
-
- Barth DS, Di S. Three-dimensional analysis of auditory-evoked potentials in rat neocortex. J Neurophysiol. 1990;64:1527–1536. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
