Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy
- PMID: 21976528
- PMCID: PMC3230070
- DOI: 10.1523/JNEUROSCI.3836-11.2011
Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy
Abstract
Because overactivation of the hypothalamic-pituitary-adrenal (HPA) axis occurs in Alzheimer's disease (AD), dysregulation of stress neuromediators may play a mechanistic role in the pathophysiology of AD. However, the effects of stress on tau phosphorylation are poorly understood, and the relationship between corticosterone and corticotropin-releasing factor (CRF) on both β-amyloid (Aβ) and tau pathology remain unclear. Therefore, we first established a model of chronic stress, which exacerbates Aβ accumulation in Tg2576 mice and then extended this stress paradigm to a tau transgenic mouse model with the P301S mutation (PS19) that displays tau hyperphosphorylation, insoluble tau inclusions and neurodegeneration. We show for the first time that both Tg2576 and PS19 mice demonstrate a heightened HPA stress profile in the unstressed state. In Tg2576 mice, 1 month of restraint/isolation (RI) stress increased Aβ levels, suppressed microglial activation, and worsened spatial and fear memory compared with nonstressed mice. In PS19 mice, RI stress promoted tau hyperphosphorylation, insoluble tau aggregation, neurodegeneration, and fear-memory impairments. These effects were not mimicked by chronic corticosterone administration but were prevented by pre-stress administration of a CRF receptor type 1 (CRF(1)) antagonist. The role for a CRF(1)-dependent mechanism was further supported by the finding that mice overexpressing CRF had increased hyperphosphorylated tau compared with wild-type littermates. Together, these results implicate HPA dysregulation in AD neuropathogenesis and suggest that prolonged stress may increase Aβ and tau hyperphosphorylation. These studies also implicate CRF in AD pathophysiology and suggest that pharmacological manipulation of this neuropeptide may be a potential therapeutic strategy for AD.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

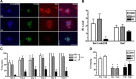
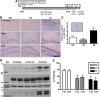
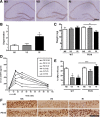
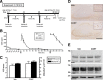
Comment in
-
Neurodegenerative disease: CRF is the culprit.Nat Rev Neurosci. 2011 Nov 3;12(12):704. doi: 10.1038/nrn3139. Nat Rev Neurosci. 2011. PMID: 22048064 No abstract available.
References
-
- Alfarez DN, Joëls M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. Eur J Neurosci. 2003;17:1928–1934. - PubMed
-
- Avila J, Wandosell F, Hernández F. Role of glycogen synthase kinase-3 in Alzheimer's disease pathogenesis and glycogen synthase kinase-3 inhibitors. Expert Rev Neurother. 2010;10:703–710. - PubMed
-
- Aznar S, Knudsen GM. Depression and Alzheimer's disease: is stress the initiating factor in a common neuropathological cascade? J Alzheimers Dis. 2011;23:177–193. - PubMed
-
- Bachman DL, Wolf PA, Linn R, Knoefel JE, Cobb J, Belanger A, D'Agostino RB, White LR. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992;42:115–119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
