The cargo-binding domain of transportin 3 is required for lentivirus nuclear import
- PMID: 21976643
- PMCID: PMC3233122
- DOI: 10.1128/JVI.05384-11
The cargo-binding domain of transportin 3 is required for lentivirus nuclear import
Abstract
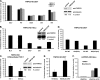
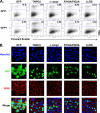
Lentiviruses, unlike the gammaretroviruses, are able to infect nondividing cells by transiting through nuclear pores to access the host genomic DNA. Several nuclear import and nuclear pore components have been implicated as playing a role in nuclear import, including transportin 3 (TNPO3), a member of the importin-β family of nuclear import proteins. We demonstrated that TNPO3 was required by several lentiviruses, with simian immunodeficiency virus mac239 (SIVmac239) and equine infectious anemia virus (EIAV) the most dependent and human immunodeficiency virus type 1 (HIV-1) and feline immunodeficiency virus (FIV) the least. Analysis of HIV-1/SIVmac239 chimeric viruses showed that dependence on TNPO3 mapped to the SIVmac239 capsid. Mutation of a single amino acid, A76V in the SIVmac239 capsid, rendered the virus TNPO3 independent and resistant to mCPSF6-358, a truncated splicing factor that prevents HIV-1 nuclear import. Using a complementation assay based on 293T cells that express a TNPO3-targeted short hairpin RNA (shRNA), we showed that the Drosophila TNPO3 homologue can substitute for its human counterpart and that it mapped a key functional domain of TNPO3 to the carboxy-terminal cargo-binding domain. Within the cargo-binding domain, two hydrophobic motifs were required for TNPO3-dependent infection. The mutated TNPO3 proteins maintained their ability to localize to the nucleus, suggesting that their inability to restore lentivirus infection resulted from an inability to bind to a host or viral cargo protein.
Figures






References
-
- Bayliss R., Littlewood T., Stewart M. 2000. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell 102:99–108 - PubMed
-
- Bouyac-Bertoia M., et al. 2001. HIV-1 infection requires a functional integrase NLS. Mol. Cell 7:1025–1035 - PubMed
-
- Brass A. L., et al. 2008. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319:921–926 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

