PEX5 protein binds monomeric catalase blocking its tetramerization and releases it upon binding the N-terminal domain of PEX14
- PMID: 21976670
- PMCID: PMC3220454
- DOI: 10.1074/jbc.M111.287201
PEX5 protein binds monomeric catalase blocking its tetramerization and releases it upon binding the N-terminal domain of PEX14
Abstract
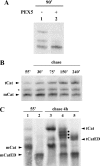
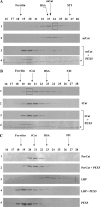
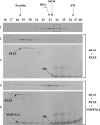
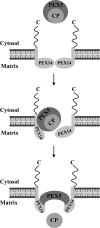
Newly synthesized peroxisomal matrix proteins are targeted to the organelle by PEX5. PEX5 has a dual role in this process. First, it acts as a soluble receptor recognizing these proteins in the cytosol. Subsequently, at the peroxisomal docking/translocation machinery, PEX5 promotes their translocation across the organelle membrane. Despite significant advances made in recent years, several aspects of this pathway remain unclear. Two important ones regard the formation and disruption of the PEX5-cargo protein interaction in the cytosol and at the docking/translocation machinery, respectively. Here, we provide data on the interaction of PEX5 with catalase, a homotetrameric enzyme in its native state. We found that PEX5 interacts with monomeric catalase yielding a stable protein complex; no such complex was detected with tetrameric catalase. Binding of PEX5 to monomeric catalase potently inhibits its tetramerization, a property that depends on domains present in both the N- and C-terminal halves of PEX5. Interestingly, the PEX5-catalase interaction is disrupted by the N-terminal domain of PEX14, a component of the docking/translocation machinery. One or two of the seven PEX14-binding diaromatic motifs present in the N-terminal half of PEX5 are probably involved in this phenomenon. These results suggest the following: 1) catalase domain(s) involved in the interaction with PEX5 are no longer accessible upon tetramerization of the enzyme; 2) the catalase-binding interface in PEX5 is not restricted to its C-terminal peroxisomal targeting sequence type 1-binding domain and also involves PEX5 N-terminal domain(s); and 3) PEX14 participates in the cargo protein release step.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

