Polyphosphate is a cofactor for the activation of factor XI by thrombin
- PMID: 21976677
- PMCID: PMC3245215
- DOI: 10.1182/blood-2011-07-368811
Polyphosphate is a cofactor for the activation of factor XI by thrombin
Abstract
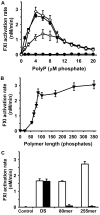
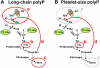
Factor XI deficiency is associated with a bleeding diathesis, but factor XII deficiency is not, indicating that, in normal hemostasis, factor XI must be activated in vivo by a protease other than factor XIIa. Several groups have identified thrombin as the most likely activator of factor XI, although this reaction is slow in solution. Although certain nonphysiologic anionic polymers and surfaces have been shown to enhance factor XI activation by thrombin, the physiologic cofactor for this reaction is uncertain. Activated platelets secrete the highly anionic polymer polyphosphate, and our previous studies have shown that polyphosphate has potent procoagulant activity. We now report that polyphosphate potently accelerates factor XI activation by α-thrombin, β-thrombin, and factor XIa and that these reactions are supported by polyphosphate polymers of the size secreted by activated human platelets. We therefore propose that polyphosphate is a natural cofactor for factor XI activation in plasma that may help explain the role of factor XI in hemostasis and thrombosis.
Figures




Comment in
-
A cofactor for factor XI activation.Blood. 2011 Dec 22;118(26):6730-1. doi: 10.1182/blood-2011-10-385336. Blood. 2011. PMID: 22194395 No abstract available.
References
-
- Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. - PubMed
-
- Ragni MV, Sinha D, Seaman F, Lewis JH, Spero JA, Walsh PN. Comparison of bleeding tendency, factor XI coagulant activity, and factor XI antigen in 25 factor XI-deficient kindreds. Blood. 1985;65(3):719–724. - PubMed
-
- Salomon O, Steinberg DM, Seligshon U. Variable bleeding manifestations characterize different types of surgery in patients with severe factor XI deficiency enabling parsimonious use of replacement therapy. Haemophilia. 2006;12(5):490–493. - PubMed
-
- Rosenthal RL, Dreskin OH, Rosenthal N. Plasma thromboplastin antecedent (PTA) deficiency; clinical, coagulation, therapeutic and hereditary aspects of a new hemophilia-like disease. Blood. 1955;10(2):120–131. - PubMed
-
- Asakai R, Chung DW, Davie EW, Seligsohn U. Factor XI deficiency in Ashkenazi Jews in Israel. N Engl J Med. 1991;325(3):153–158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

