Hereditary pituitary hyperplasia with infantile gigantism
- PMID: 21976722
- PMCID: PMC3232621
- DOI: 10.1210/jc.2011-1401
Hereditary pituitary hyperplasia with infantile gigantism
Abstract
Context: We report hereditary pituitary hyperplasia.
Objective: The objective of the study was to describe the results of the clinical and laboratory analysis of this rare instance of hereditary pituitary hyperplasia.
Design: The study is a retrospective analysis of three cases from one family.
Setting: The study was conducted at the National Institutes of Health, a tertiary referral center.
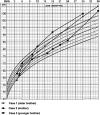
Patients: A mother and both her sons had very early-onset gigantism associated with high levels of serum GH and prolactin.
Interventions: The condition was treated by total hypophysectomy.
Main outcome measure(s): We performed clinical, pathological, and molecular evaluations, including evaluation basal and provocative endocrine testing, neuroradiological assessment, and assessment of the pituitary tissue by microscopic evaluation, immunohistochemistry, and electron microscopy.
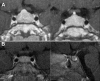
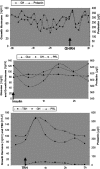
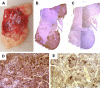
Results: All three family members had very early onset of gigantism associated with abnormally high serum levels of GH and prolactin. Serum GHRH levels were not elevated in either of the boys. The clinical, radiographic, surgical, and histological findings indicated mammosomatotroph hyperplasia. The pituitary gland of both boys revealed diffuse mammosomatotroph hyperplasia of the entire pituitary gland without evidence of adenoma. Prolactin and GH were secreted by the same cells within the same secretory granules. Western blot and immunohistochemistry demonstrated expression of GHRH in clusters of cells distributed throughout the hyperplastic pituitary of both boys.
Conclusions: This hereditary condition seems to be a result of embryonic pituitary maldevelopment with retention and expansion of the mammosomatotrophs. The findings suggest that it is caused by paracrine or autocrine pituitary GHRH secretion during pituitary development.
Figures


References
-
- Mayo KE, Hammer RE, Swanson LW, Brinster RL, Rosenfeld MG, Evans RM. 1988. Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Mol Endocrinol 2:606–612 - PubMed
-
- Asa SL, Kovacs K, Stefaneanu L, Horvath E, Billestrup N, Gonzalez-Manchon C, Vale W. 1990. Pituitary mammosomatotroph adenomas develop in old mice transgenic for growth hormone-releasing hormone. Proc Soc Exp Biol Med 193:232–235 - PubMed
-
- Espiner EA, Carter TA, Abbott GD, Wrightson P. 1981. Pituitary gigantism in a 31 month old girl: endocrine studies and successful response to hypophysectomy. J Endocrinol Invest 4:445–450 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

