Structural and functional roles of deamidation of N146 and/or truncation of NH2- or COOH-termini in human αB-crystallin
- PMID: 21976952
- PMCID: PMC3185027
Structural and functional roles of deamidation of N146 and/or truncation of NH2- or COOH-termini in human αB-crystallin
Abstract
Purpose: The purpose of the study was to determine the relative effects of deamidation and/or truncation on the structural and functional properties of αB-crystallin.
Methods: Using wild-type (WT) αB-crystallin and the αB deamidated mutant (i.e., αB N146D), we generated NH(2)-terminal domain deleted (residues no. 1-66; αB-NT), deamidated plus NH(2)-terminal domain deleted (αB N146D-NT), COOH-terminal extension deleted (residues no. 151-175; αB-CT), and deamidated plus COOH-terminal extension deleted (αB N146D-CT) mutants. All of the proteins were purified and their structural and functional (chaperone activity with insulin as target protein) properties were determined and compared to WT αB-crystallin.
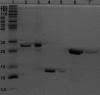
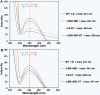
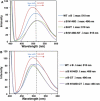
Results: The desired deletions in the αB-crystallin mutants were confirmed by DNA sequencing and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometric analysis. The homomers of αB-CT and its deamidated form (αB N146D-CT) became water insoluble, whereas the αB N146D, αB-NT, and αB N146D-NT species remained water-soluble. CD spectroscopic studies revealed that the mutants with deletion of NH(2)- or COOH-termini or deamidation showed increased β-sheet and decreased α-helical contents with the exception of αB N146D-CT, which showed a substantial increase in α-helix and decrease in β-sheet content. Results of intrinsic Trp fluorescence suggested little change in Trp microenvironment of αB N146D relative to WT αB, but substantial alterations on deletion of COOH-terminal extension or a combination of this deletion plus deamidation. Hydrophobic binding studies using the hydrophobic probe 8-anilino-1-naphthalene sulfonate (ANS) showed that, relative to WT αB structure, the N146 deamidation, COOH-terminal extension deletion or a combination of this deamidation and deletion resulted in a relatively compact structure whereas the NH(2)-terminal domain deletion and a combination of this deletion plus deamidation resulted in a relaxed structure. All the αB mutants showed higher molecular mass ranging between 1.2×10(6) to 5.4×10(6) Da, relative to WT αB which had a molecular mass of 5.8×10(5) Da. Chaperone activity across all αB species decreased in the following order: WTαB > αB N146D-CT > αB N146D-NT > αB-NT > αB-CT > αB N146D. Specifically, substantial losses in chaperone activity (only 10% to 20% protection) were seen in αB N146D, αB-NT, and αB-CT. However, in the species with the combination of deamidation plus NH(2)- or COOH-terminal deletion, the percent protection was about 24% in αB N146D-NT and about 40% in αB N146D-CT.
Conclusions: Although all mutants formed oligomers even after deamidation, on deletion of either NH(2)-terminal domain or COOH-terminal extension or a combination of these deletions and deamidation, their structural properties were substantially altered. The results suggested that the NH(2)-terminal domain is relatively more important than the COOH-terminal extension for the chaperone function of αB. The non-deamidated N146 residue, NH(2)-terminal domain and COOH-terminal extension are also of critical importance to the maintenance of αB-crystallin chaperone activity.
Figures



References
-
- Bloemendal H, de Jong WW, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–85. - PubMed
-
- Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26:78–98. - PubMed
-
- Bhat SP, Nagineni CN. αB subunit of lens-specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem Biophys Res Commun. 1989;158:319–25. - PubMed
-
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin-small heat-shock protein superfamily. Int J Biol Macromol. 1998;22:151–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
