Meta-metallation of N,N-dimethylaniline: Contrasting direct sodium-mediated zincation with indirect sodiation-dialkylzinc co-complexation
- PMID: 21977208
- PMCID: PMC3182433
- DOI: 10.3762/bjoc.7.144
Meta-metallation of N,N-dimethylaniline: Contrasting direct sodium-mediated zincation with indirect sodiation-dialkylzinc co-complexation
Abstract
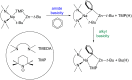
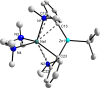
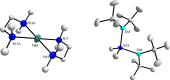
Previously we reported that direct zincation of N,N-dimethylaniline by the mixed-metal zincate reagent 1 ((TMEDA)Na(TMP)(t-Bu)Zn(t-Bu)) surprisingly led to meta-metallation (zincation) of the aniline, as manifested in the crystalline complex 2 ((TMEDA)Na(TMP)(m-C(6)H(4)-NMe(2))Zn(t-Bu)), and that iodination of these isolated crystals produced the meta-isomer N,N-dimethyl-3-iodoaniline quantitatively. Completing the study here we find that treating the reaction solution with iodine produces a 72% conversion and results in a mixture of regioisomers of N,N-dimethyliodoaniline, with the meta-isomer still the major product (ortho:meta:para ratio, 6:73:21), as determined by NMR. In contrast to this bimetallic method, sodiation of N,N-dimethylaniline with n-BuNa produced the dimeric, ortho-sodiated complex 3 (((TMEDA)Na(o-C(6)H(4)-NMe(2)))(2)), as characterised by X-ray crystallography and NMR. No regioisomers were observed in the reaction solution. Introducing t-Bu(2)Zn to this reaction solution afforded a cocrystalline product in the solid-state, composed of the bis-anilide 4 ((TMEDA)Na(o-C(6)H(4)-NMe(2))(2)Zn(t-Bu)) and the Me(2)N-C cleavage product 5 ({(TMEDA)(2)Na}(+){(t-Bu(2)Zn)(2)(µ-NMe(2))}(-)), which was characterised by X-ray crystallography. NMR studies of the reaction mixture that produces 4 and 5 revealed one additional species, but the mixture as a whole contained only ortho-species and a trace amount of para-species as established by iodine quenching. In an indirect variation of the bimetallic reaction, TMP(H) was added at room temperature to the reaction mixture that afforded 4 and 5. This gave the crystalline product 6 ((TMEDA)Na(TMP)(o-C(6)H(4)-NMe(2))Zn(t-Bu)), the ortho-isomer of the meta-complex 2, as determined from X-ray crystallographic and NMR data. Monitoring the regioselectivity of the reaction by iodination revealed a 16.6:1.6:1.0 ortho:meta:para ratio. Interestingly, when the TMP(H) containing solution was heated under reflux for 18 hours more meta-isomer was produced (corresponding ratio 3.7:4.2:1.0). It is likely that this change has its origin in a retro reaction that produces the original base 1 as an intermediate. Theoretical calculations at the DFT level using the B3LYP method and the 6-311G** basis set were used to probe the energetics of both monometallic and bimetallic systems. In accord with the experimental results, it was found that ortho-metallation was favoured by sodiation; whereas meta- (closely followed by para-) metallation was favoured by direct sodium-mediated zincation.
Keywords: alkali metal; crystal structure; isomerisation; metallation; zincation.
Figures






Similar articles
-
Structural basis for regioisomerization in the alkali-metal-mediated zincation (AMMZn) of trifluoromethyl benzene by isolation of kinetic and thermodynamic intermediates.J Am Chem Soc. 2010 Jul 14;132(27):9480-7. doi: 10.1021/ja1038598. J Am Chem Soc. 2010. PMID: 20568749 Free PMC article.
-
Synthetic and structural insights into the zincation of toluene: direct synergic ring metallation versus indirect nonsynergic lateral metallation.Chemistry. 2009;15(15):3800-7. doi: 10.1002/chem.200801928. Chemistry. 2009. PMID: 19229931
-
Synthetic and reactivity studies of hetero-tri-anionic sodium zincates.Dalton Trans. 2016 Apr 14;45(14):6222-33. doi: 10.1039/c5dt04329h. Epub 2015 Dec 15. Dalton Trans. 2016. PMID: 26666657
-
Evaluating cis-2,6-dimethylpiperidide (cis-DMP) as a base component in lithium-mediated zincation chemistry.Chemistry. 2013 Sep 27;19(40):13492-503. doi: 10.1002/chem.201301180. Epub 2013 Aug 19. Chemistry. 2013. PMID: 23955639 Free PMC article.
-
Heterobimetallic metallation studies of N,N-dimethylphenylethylamine (DMPEA): benzylic C-H bond cleavage/dimethylamino capture or intact DMPEA complex.Dalton Trans. 2015 Mar 28;44(12):5875-87. doi: 10.1039/c5dt00247h. Dalton Trans. 2015. PMID: 25716990
Cited by
-
Rubus urticifolius Compounds with Antioxidant Activity, and Inhibition Potential against Tyrosinase, Melanin, Hyaluronidase, Elastase, and Collagenase.Pharmaceuticals (Basel). 2024 Jul 13;17(7):937. doi: 10.3390/ph17070937. Pharmaceuticals (Basel). 2024. PMID: 39065787 Free PMC article.
-
Redox-active inverse crowns for small molecule activation.Nat Chem. 2025 May;17(5):703-709. doi: 10.1038/s41557-024-01724-5. Epub 2025 Feb 17. Nat Chem. 2025. PMID: 39962192 Free PMC article.
-
Arene Activation by Calcium Hydride/Zinc Amide Equilibration.J Am Chem Soc. 2025 Aug 13;147(32):29554-29567. doi: 10.1021/jacs.5c10735. Epub 2025 Aug 2. J Am Chem Soc. 2025. PMID: 40751684 Free PMC article.
References
-
- Schlosser M, editor. Organometallics in Synthesis. 2nd ed. Chichester, U.K.: Wiley; 2002. p. Chapter 1.
-
- Gilman H, Bebb R L. J Am Chem Soc. 1939;61:109–112. doi: 10.1021/ja01870a037. - DOI
-
- Wittig G, Pockels U, Dröge H. Ber Dtsch Chem Ges. 1938;71:1903–1912. doi: 10.1002/cber.19380710922. - DOI
-
- Gschwend H W, Rodriguez H R. Org React. 1979;26:1.
LinkOut - more resources
Full Text Sources
Miscellaneous
