Novel acridone-modified MCM-41 type silica: Synthesis, characterization and fluorescence tuning
- PMID: 21977441
- PMCID: PMC3148056
- DOI: 10.3762/bjnano.2.33
Novel acridone-modified MCM-41 type silica: Synthesis, characterization and fluorescence tuning
Abstract
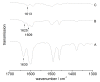
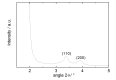
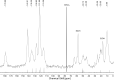
A Mobil Composition of Matter (MCM)-41 type mesoporous silica material containing N-propylacridone groups has been successfully prepared by co-condensation of an appropriate organic precursor with tetraethyl orthosilicate (TEOS) under alkaline sol-gel conditions. The resulting material was fully characterized by means of X-ray diffraction (XRD), N(2)-adsorption-desorption, transmission electron microscopy (TEM), IR and UV-vis spectroscopy, as well as (29)Si and (13)C CP-MAS NMR techniques. The material features a high inner surface area and a highly ordered two-dimensional hexagonal pore structure. The fluorescence properties of the organic chromophore can be tuned via complexation of its carbonyl group with scandium triflate, which makes the material a good candidate for solid state sensors and optics. The successful synthesis of highly ordered MCM materials through co-condensation was found to be dependent on the chemical interaction of the different precursors.
Keywords: MCM-41; acridone; co-condensation; fluorescence; scandium.
Figures







Similar articles
-
Low Release Study of Cefotaxime by Functionalized Mesoporous Silica Nanomaterials.Gels. 2022 Nov 3;8(11):711. doi: 10.3390/gels8110711. Gels. 2022. PMID: 36354619 Free PMC article.
-
Sol-Gel Synthesis of Ordered β-Cyclodextrin-Containing Silicas.Nanoscale Res Lett. 2016 Dec;11(1):174. doi: 10.1186/s11671-016-1380-2. Epub 2016 Mar 31. Nanoscale Res Lett. 2016. PMID: 27033850 Free PMC article.
-
Highly efficient luminescent hybrid materials covalently linking with europium(III) complexes via a novel fluorinated beta-diketonate ligand: synthesis, characterization and photophysical properties.Dalton Trans. 2010 Sep 14;39(34):8084-92. doi: 10.1039/c0dt00316f. Epub 2010 Jul 13. Dalton Trans. 2010. PMID: 20628688
-
Phosphonic acid functionalized ordered mesoporous material: a new and ecofriendly catalyst for one-pot multicomponent Biginelli reaction under solvent-free conditions.ACS Appl Mater Interfaces. 2014 Jan 22;6(2):933-41. doi: 10.1021/am404298a. Epub 2014 Jan 7. ACS Appl Mater Interfaces. 2014. PMID: 24372168
-
Rapid sonochemical synthesis of MCM-41 type benzene-bridged periodic mesoporous organosilicas.Ultrason Sonochem. 2014 Jan;21(1):387-94. doi: 10.1016/j.ultsonch.2013.06.014. Epub 2013 Jun 25. Ultrason Sonochem. 2014. PMID: 23835400
Cited by
-
Energy down converting organic fluorophore functionalized mesoporous silica hybrids for monolith-coated light emitting diodes.Beilstein J Org Chem. 2017 Apr 25;13:768-778. doi: 10.3762/bjoc.13.76. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 28546833 Free PMC article.
References
-
- Wang X, Chen C-C, Chen S-Y, Mou Y, Cheng S. Appl Catal, A. 2005;281:47–54. doi: 10.1016/j.apcata.2004.11.011. - DOI
-
- Hu Y, Higashimoto S, Martra G, Zhang J, Matsuoka M, Coluccia S, Anpo M. Catal Lett. 2003;90:161–163. doi: 10.1023/B:CATL.0000004111.02392.75. - DOI
-
- Zhou Z, Franz A W, Hartmann M, Seifert A, Müller T J J, Thiel W R. Chem Mater. 2008;20:4986–4992. doi: 10.1021/cm800804t. - DOI
-
- Franz A W, Zhou Z, Turdean R, Wagener A, Sarkar B, Hartmann M, Ernst S, Thiel W R, Müller T J J. Eur J Org Chem. 2009:3895–3905. doi: 10.1002/ejoc.200900332. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
