Transient modification within a pool of CD4 T cells in the maternal spleen
- PMID: 21977997
- PMCID: PMC3209567
- DOI: 10.1111/j.1365-2567.2011.03486.x
Transient modification within a pool of CD4 T cells in the maternal spleen
Abstract
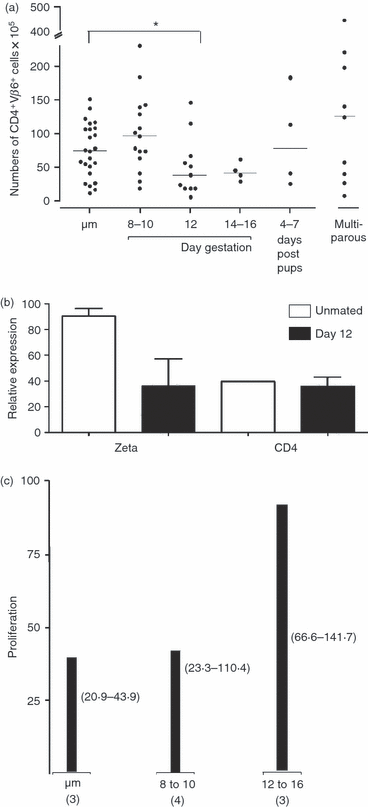
Classic models suggest maternal tolerance is dependent on regulation of fetal antigen-specific T cell responses. We hypothesize that factors unique to a particular fetal antigen-specific T cell, rather than the state of pregnancy per se, are important determinants of T cell fate during pregnancy. To investigate the fate of fetal antigen-specific CD4 T cells in the systemic circulation, we examined spleen cells in a CD4 T cell receptor transgenic mouse specific for the male antigen H-Y. We observed a transient decrease in CD4(+) Vβ6(+) cell numbers and, due to transient internalization of CD4, an increase in CD4(-) Vβ6(+) T cells. Antigen-specific in vitro responsiveness was not depressed by pregnancy. These data suggest that pregnancy supports fluidity in this particular CD4 T cell pool that may, in turn, help to meet competing requirements of maternal immune responsiveness and fetal tolerance.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures





Similar articles
-
Regional oral tolerance in transgenic 2C mice.Surgery. 2005 Aug;138(2):141-9. doi: 10.1016/j.surg.2005.05.010. Surgery. 2005. PMID: 16153420
-
All or none peripheral tolerance induction in H-Y antigen-specific TCR transgenic mice.Transpl Immunol. 1998 Jun;6(2):78-83. doi: 10.1016/s0966-3274(98)80021-x. Transpl Immunol. 1998. PMID: 9777695
-
Maturation-dependent licensing of naive T cells for rapid TNF production.PLoS One. 2010 Nov 24;5(11):e15038. doi: 10.1371/journal.pone.0015038. PLoS One. 2010. PMID: 21124839 Free PMC article.
-
IL-2 contributes to maintaining a balance between CD4+Foxp3+ regulatory T cells and effector CD4+ T cells required for immune control of blood-stage malaria infection.J Immunol. 2011 Apr 15;186(8):4862-71. doi: 10.4049/jimmunol.1003777. Epub 2011 Mar 9. J Immunol. 2011. PMID: 21389253
-
The hidden maternal-fetal interface: events involving the lymphoid organs in maternal-fetal tolerance.Int J Dev Biol. 2010;54(2-3):421-30. doi: 10.1387/ijdb.082800et. Int J Dev Biol. 2010. PMID: 19876825 Free PMC article. Review.
Cited by
-
Deficiency in CD4 T Cells Leads to Enhanced Postpartum Internal Carotid Artery Vasoconstriction in Mice: The Role of Nitric Oxide.Front Physiol. 2021 Jun 16;12:686429. doi: 10.3389/fphys.2021.686429. eCollection 2021. Front Physiol. 2021. PMID: 34220551 Free PMC article.
-
Pregnancy imparts distinct systemic adaptive immune function.Am J Reprod Immunol. 2022 Nov;88(5):e13606. doi: 10.1111/aji.13606. Epub 2022 Sep 6. Am J Reprod Immunol. 2022. PMID: 35989229 Free PMC article.
-
Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone.J Immunol. 2019 May 1;202(9):2585-2608. doi: 10.4049/jimmunol.1801350. Epub 2019 Mar 27. J Immunol. 2019. PMID: 30918041 Free PMC article. Clinical Trial.
-
Demystifying animal models of adverse pregnancy outcomes: touching bench and bedside.Am J Reprod Immunol. 2013 Jun;69(6):567-84. doi: 10.1111/aji.12102. Epub 2013 Feb 28. Am J Reprod Immunol. 2013. PMID: 23448345 Free PMC article.
-
The Cellular Transcriptome in the Maternal Circulation During Normal Pregnancy: A Longitudinal Study.Front Immunol. 2019 Dec 17;10:2863. doi: 10.3389/fimmu.2019.02863. eCollection 2019. Front Immunol. 2019. PMID: 31921132 Free PMC article. Clinical Trial.
References
-
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. - PubMed
-
- Zenclussen AC, Gerlof K, Zenclussen ML, et al. Regulatory T cells induce a privileged tolerant microenvironment at the fetal–maternal interface. Eur J Immunol. 2006;36:82–94. - PubMed
-
- Vacchio MS, Hodes RJ. Fetal expression of Fas ligand is necessary and sufficient for induction of CD8 T cell tolerance to the fetal antigen H-Y during pregnancy. J Immunol. 2005;174:4657–61. - PubMed
-
- Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol. 2004;5:266–71. - PubMed
-
- Bonney EA, Matzinger P. The maternal immune system's interaction with circulating fetal cells. J Immunol. 1997;158:40–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

