RACK1, A multifaceted scaffolding protein: Structure and function
- PMID: 21978545
- PMCID: PMC3195729
- DOI: 10.1186/1478-811X-9-22
RACK1, A multifaceted scaffolding protein: Structure and function
Abstract
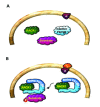
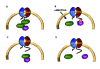
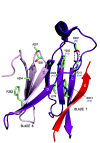
The Receptor for Activated C Kinase 1 (RACK1) is a member of the tryptophan-aspartate repeat (WD-repeat) family of proteins and shares significant homology to the β subunit of G-proteins (Gβ). RACK1 adopts a seven-bladed β-propeller structure which facilitates protein binding. RACK1 has a significant role to play in shuttling proteins around the cell, anchoring proteins at particular locations and in stabilising protein activity. It interacts with the ribosomal machinery, with several cell surface receptors and with proteins in the nucleus. As a result, RACK1 is a key mediator of various pathways and contributes to numerous aspects of cellular function. Here, we discuss RACK1 gene and structure and its role in specific signaling pathways, and address how posttranslational modifications facilitate subcellular location and translocation of RACK1. This review condenses several recent studies suggesting a role for RACK1 in physiological processes such as development, cell migration, central nervous system (CN) function and circadian rhythm as well as reviewing the role of RACK1 in disease.
Figures




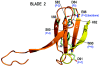

References
-
- Fong HK, Hurley JB, Hopkins RS, Miake-Lye R, Johnson MS, Doolittle RF, Simon MI. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci USA. 1986;83:2162–2166. doi: 10.1073/pnas.83.7.2162. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

