Voltage-dependent conformational changes in connexin channels
- PMID: 21978595
- PMCID: PMC3367129
- DOI: 10.1016/j.bbamem.2011.09.019
Voltage-dependent conformational changes in connexin channels
Abstract
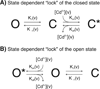
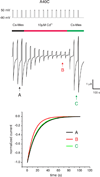
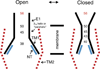
Channels formed by connexins display two distinct types of voltage-dependent gating, termed V(j)- or fast-gating and loop- or slow-gating. Recent studies, using metal bridge formation and chemical cross-linking have identified a region within the channel pore that contributes to the formation of the loop-gate permeability barrier. The conformational changes are remarkably large, reducing the channel pore diameter from 15 to 20Å to less than 4Å. Surprisingly, the largest conformational change occurs in the most stable region of the channel pore, the 3(10) or parahelix formed by amino acids in the 42-51 segment. The data provide a set of positional constraints that can be used to model the structure of the loop-gate closed state. Less is known about the conformation of the V(j)-gate closed state. There appear to be two different mechanisms; one in which conformational changes in channel structure are linked to a voltage sensor contained in the N-terminus of Cx26 and Cx32 and a second in which the C-terminus of Cx43 and Cx40 may act either as a gating particle to block the channel pore or alternatively to stabilize the closed state. The later mechanism utilizes the same domains as implicated in effecting pH gating of Cx43 channels. It is unclear if the two V(j)-gating mechanisms are related or if they represent different gating mechanisms that operate separately in different subsets of connexin channels. A model of the V(j)-closed state of Cx26 hemichannel that is based on the X-ray structure of Cx26 and electron crystallographic structures of a Cx26 mutation suggests that the permeability barrier for V(j)-gating is formed exclusively by the N-terminus, but recent information suggests that this conformation may not represent a voltage-closed state. Closed state models are considered from a thermodynamic perspective based on information from the 3.5Å Cx26 crystal structure and molecular dynamics (MD) simulations. The applications of computational and experimental methods to define the path of allosteric molecular transitions that link the open and closed states are discussed. This article is part of a Special Issue entitled: The Communicating junctions, composition, structure and characteristics.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

