Complement factor H binds malondialdehyde epitopes and protects from oxidative stress
- PMID: 21979047
- PMCID: PMC4826616
- DOI: 10.1038/nature10449
Complement factor H binds malondialdehyde epitopes and protects from oxidative stress
Abstract
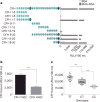
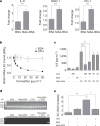
Oxidative stress and enhanced lipid peroxidation are linked to many chronic inflammatory diseases, including age-related macular degeneration (AMD). AMD is the leading cause of blindness in Western societies, but its aetiology remains largely unknown. Malondialdehyde (MDA) is a common lipid peroxidation product that accumulates in many pathophysiological processes, including AMD. Here we identify complement factor H (CFH) as a major MDA-binding protein that can block both the uptake of MDA-modified proteins by macrophages and MDA-induced proinflammatory effects in vivo in mice. The CFH polymorphism H402, which is strongly associated with AMD, markedly reduces the ability of CFH to bind MDA, indicating a causal link to disease aetiology. Our findings provide important mechanistic insights into innate immune responses to oxidative stress, which may be exploited in the prevention of and therapy for AMD and other chronic inflammatory diseases.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Comment in
-
Molecular medicine: Defence against oxidative damage.Nature. 2011 Oct 5;478(7367):42-3. doi: 10.1038/478042a. Nature. 2011. PMID: 21979040 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

