Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model
- PMID: 21979052
- PMCID: PMC3191865
- DOI: 10.1038/nature10485
Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model
Abstract
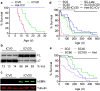
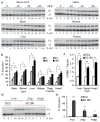
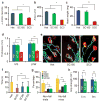
Spinal muscular atrophy (SMA) is a motor neuron disease and the leading genetic cause of infant mortality; it results from loss-of-function mutations in the survival motor neuron 1 (SMN1) gene. Humans have a paralogue, SMN2, whose exon 7 is predominantly skipped, but the limited amount of functional, full-length SMN protein expressed from SMN2 cannot fully compensate for a lack of SMN1. SMN is important for the biogenesis of spliceosomal small nuclear ribonucleoprotein particles, but downstream splicing targets involved in pathogenesis remain elusive. There is no effective SMA treatment, but SMN restoration in spinal cord motor neurons is thought to be necessary and sufficient. Non-central nervous system (CNS) pathologies, including cardiovascular defects, were recently reported in severe SMA mouse models and patients, reflecting autonomic dysfunction or direct effects in cardiac tissues. Here we compared systemic versus CNS restoration of SMN in a severe mouse model. We used an antisense oligonucleotide (ASO), ASO-10-27, that effectively corrects SMN2 splicing and restores SMN expression in motor neurons after intracerebroventricular injection. Systemic administration of ASO-10-27 to neonates robustly rescued severe SMA mice, much more effectively than intracerebroventricular administration; subcutaneous injections extended the median lifespan by 25 fold. Furthermore, neonatal SMA mice had decreased hepatic Igfals expression, leading to a pronounced reduction in circulating insulin-like growth factor 1 (IGF1), and ASO-10-27 treatment restored IGF1 to normal levels. These results suggest that the liver is important in SMA pathogenesis, underscoring the importance of SMN in peripheral tissues, and demonstrate the efficacy of a promising drug candidate.
Figures

Comment in
-
Antisense therapeutics: Systemic reawakening of a silent gene to improve survival in SMA.Nat Rev Drug Discov. 2011 Dec 1;10(12):900. doi: 10.1038/nrd3608. Nat Rev Drug Discov. 2011. PMID: 22129985 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

