Evolution under environmental stress at macro- and microscales
- PMID: 21979157
- PMCID: PMC3227405
- DOI: 10.1093/gbe/evr052
Evolution under environmental stress at macro- and microscales
Abstract
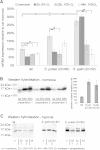
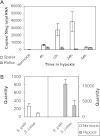
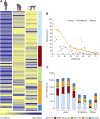
Environmental stress has played a major role in the evolution of living organisms (Hoffman AA, Parsons PA. 1991. Evolutionary genetics and environmental stress. Oxford: Oxford University Press; Parsons PA. 2005. Environments and evolution: interactions between stress, resource inadequacy, and energetic efficiency. Biol Rev Camb Philos Soc. 80:589-610). This is reflected by the massive and background extinctions in evolutionary time (Nevo E. 1995a. Evolution and extinction. Encyclopedia of Environmental Biology. New York: Academic Press, Inc. 1:717-745). The interaction between organism and environment is central in evolution. Extinction ensues when organisms fail to change and adapt to the constantly altering abiotic and biotic stressful environmental changes as documented in the fossil record. Extreme environmental stress causes extinction but also leads to evolutionary change and the origination of new species adapted to new environments. I will discuss a few of these global, regional, and local stresses based primarily on my own research programs. These examples will include the 1) global regional and local experiment of subterranean mammals; 2) regional experiment of fungal life in the Dead Sea; 3) evolution of wild cereals; 4) "Evolution Canyon"; 5) human brain evolution, and 6) global warming.
Figures





References
-
- Arieli R, Arieli M, Heth G, Nevo E. Adaptive respiratory variation in four chromosomal species of mole rats. Experientia. 1984;40:512–514. - PubMed
-
- Arieli R, Heth G, Nevo E, Hoch D. Hematocrit and hemoglobin concentration in four chromosomal species and some isolated populations of actively speciating subterranean mole rats in Israel. Experientia. 1986;42:441–443. - PubMed
-
- Arieli R, Heth G, Nevo E, Zamir Y, Neutra O. Adaptive heart and breathing frequencies in four ecologically differentiating chromosomal species of mole rats in Israel. Experientia. 1986;42:131–133.
-
- Arieli R, Nevo E. Hypoxic survival differs between two mole rat species (Spalax ehrenbergi) of humid and arid habitats. Comp Biochem Physiol A Comp Physiol. 1991;3:543–545. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

