Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations
- PMID: 21979374
- PMCID: PMC3205584
- DOI: 10.1101/gad.17620611
Opposing roles of NF-κB in anti-cancer treatment outcome unveiled by cross-species investigations
Abstract
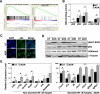
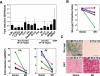
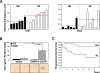
In malignancies, enhanced nuclear factor-κB (NF-κB) activity is largely viewed as an oncogenic property that also confers resistance to chemotherapy. Recently, NF-κB has been postulated to participate in a senescence-associated and possibly senescence-reinforcing cytokine response, thereby suggesting a tumor-restraining role for NF-κB. Using a mouse lymphoma model and analyzing transcriptome and clinical data from lymphoma patients, we show here that therapy-induced senescence presents with and depends on active NF-κB signaling, whereas NF-κB simultaneously promotes resistance to apoptosis. Further characterization and genetic engineering of primary mouse lymphomas according to distinct NF-κB-related oncogenic networks reminiscent of diffuse large B-cell lymphoma (DLBCL) subtypes guided us to identify Bcl2-overexpressing germinal center B-cell-like (GCB) DLBCL as a clinically relevant subgroup with significantly superior outcome when NF-κB is hyperactive. Our data illustrate the power of cross-species investigations to functionally test genetic mechanisms in transgenic mouse tumors that recapitulate distinct features of the corresponding human entity, and to ultimately use the mouse model-derived genetic information to redefine novel, clinically relevant patient subcohorts.
Figures

Comment in
-
Senescence. NF-κB shows its beneficial side.Nat Rev Cancer. 2011 Oct 28;11(12):832-3. doi: 10.1038/nrc3168. Nat Rev Cancer. 2011. PMID: 22020169 No abstract available.
-
The Two Faces of NF-κB Signaling in Cancer Development and Therapy.Cancer Cell. 2011 Nov 15;20(5):556-8. doi: 10.1016/j.ccr.2011.10.026. Cancer Cell. 2011. PMID: 22094250 Free PMC article.
References
-
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018 - PubMed
-
- Adams JM, Harris AW, Pinkert CA, Corcoran LM, Alexander WS, Cory S, Palmiter RD, Brinster RL 1985. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318: 533–538 - PubMed
-
- Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, Kluin PM 2011. Double-hit B-cell lymphomas. Blood 117: 2319–2331 - PubMed
-
- Ben-Neriah Y, Karin M 2011. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol 12: 715–723 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
