Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress
- PMID: 21979438
- PMCID: PMC3380608
- DOI: 10.1161/ATVBAHA.111.236570
Spatial distribution and mechanical function of elastin in resistance arteries: a role in bearing longitudinal stress
Abstract
Objective: Despite the role that extracellular matrix (ECM) plays in vascular signaling, little is known of the complex structural arrangement between specific ECM proteins and vascular smooth muscle cells. Our objective was to examine the hypothesis that adventitial elastin fibers are dominant in vessels subject to longitudinal stretch.
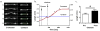
Methods and results: Cremaster muscle arterioles were isolated, allowed to develop spontaneous tone, and compared with small cerebral arteries. 3D confocal microscopy was used to visualize ECM within the vessel wall. Pressurized arterioles were fixed and stained with Alexa 633 hydrazide (as a nonselective ECM marker), anti-elastin, or anti-type 1 collagen antibody and a fluorescent nuclear stain. Exposure of cremaster muscle arterioles to elastase for 5 minutes caused an irreversible lengthening of the vessel segment that was not observed in cerebral arteries. Longitudinal elastin fibers were demonstrated on cremaster muscle arterioles using 3D imaging but were confirmed to be absent in cerebral vessels. The fibers were also distinct from type I collagen fibers and were degraded by elastase treatment.
Conclusions: These results indicate the importance of elastin in bearing longitudinal stress in the arteriolar wall and that these fibers constrain vascular smooth muscle cells. Differences between skeletal muscle and cerebral small arteries may reflect differences in the local mechanical environment, such as exposure to longitudinal stretch.
Figures

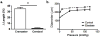




Similar articles
-
Mechanotransduction via the elastin-laminin receptor (ELR) in resistance arteries.J Biomech. 2003 May;36(5):645-52. doi: 10.1016/s0021-9290(02)00442-6. J Biomech. 2003. PMID: 12694994
-
Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit.J Physiol. 2009 Jun 15;587(Pt 12):3025-44. doi: 10.1113/jphysiol.2009.169920. Epub 2009 Apr 9. J Physiol. 2009. PMID: 19359368 Free PMC article.
-
Imaging and modeling of acute pressure-induced changes of collagen and elastin microarchitectures in pig and human resistance arteries.Am J Physiol Heart Circ Physiol. 2017 Jul 1;313(1):H164-H178. doi: 10.1152/ajpheart.00110.2017. Epub 2017 Apr 21. Am J Physiol Heart Circ Physiol. 2017. PMID: 28432057
-
Small Artery Elastin Distribution and Architecture-Focus on Three Dimensional Organization.Microcirculation. 2016 Nov;23(8):614-620. doi: 10.1111/micc.12294. Microcirculation. 2016. PMID: 27362628 Review.
-
Review of the Techniques Used for Investigating the Role Elastin and Collagen Play in Arterial Wall Mechanics.IEEE Rev Biomed Eng. 2021;14:256-269. doi: 10.1109/RBME.2020.3005448. Epub 2021 Jan 22. IEEE Rev Biomed Eng. 2021. PMID: 32746366 Review.
Cited by
-
Intraluminal pressure elevates intracellular calcium and contracts CNS pericytes: Role of voltage-dependent calcium channels.Proc Natl Acad Sci U S A. 2023 Feb 28;120(9):e2216421120. doi: 10.1073/pnas.2216421120. Epub 2023 Feb 21. Proc Natl Acad Sci U S A. 2023. PMID: 36802432 Free PMC article.
-
Developmental diversity and unique sensitivity to injury of lung endothelial subtypes during postnatal growth.iScience. 2023 Jan 31;26(3):106097. doi: 10.1016/j.isci.2023.106097. eCollection 2023 Mar 17. iScience. 2023. PMID: 36879800 Free PMC article.
-
Diverse homeostatic and immunomodulatory roles of immune cells in the developing mouse lung at single cell resolution.Elife. 2020 Jun 2;9:e56890. doi: 10.7554/eLife.56890. Elife. 2020. PMID: 32484158 Free PMC article.
-
Mechanisms of the inward remodeling process in resistance vessels: is the actin cytoskeleton involved?Microcirculation. 2014 Apr;21(3):219-29. doi: 10.1111/micc.12105. Microcirculation. 2014. PMID: 24635509 Free PMC article. Review.
-
Vascular elastic fiber heterogeneity in health and disease.Curr Opin Hematol. 2020 May;27(3):190-196. doi: 10.1097/MOH.0000000000000578. Curr Opin Hematol. 2020. PMID: 32141894 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

