Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia
- PMID: 21979451
- PMCID: PMC3288729
- DOI: 10.1007/s00429-011-0351-8
Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia
Abstract
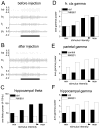
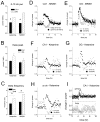
The proper organization and function of GABAergic interneuron networks is essential for many cognitive processes and abnormalities in these systems have been documented in schizophrenic patients. The memory function of the hippocampus depends on two major patterns of oscillations in the theta and gamma ranges, both requiring the intact functioning of the network of fast-firing interneurons expressing parvalbumin. We examined the ability of acute and chronic administration of NMDA receptor (NMDA-R) antagonists to recapitulate the oscillatory dysfunctions observed in schizophrenia. In freely moving rats, acute injection of MK801 or ketamine increased gamma power in both CA1 and dentate gyrus of the hippocampus. Theta peak shifted to higher frequencies whereas the average 5-10 Hz theta power decreased by 24% in CA1 and remained high in the dentate gyrus. Strong increase in CA1 gamma and decrease in theta power triggered by brainstem stimulation were found under urethane anesthesia. In contrast to acute experiments, chronic administration of ketamine caused a steady decline in both gamma and theta oscillations, 2-4 weeks after treatment. A further important difference between the two models was that the effects of acute injection were more robust than the changes after chronic treatment. Chronic administration of ketamine also leads to decrease in the number of detectable parvalbumin interneurons. Histological examination of interindividual differences indicated, however, that within the ketamine treated group a further decrease in parvalbumin neurons correlated with strengthening of oscillations. The findings are consistent with abnormalities of oscillations in human schizophrenia and further validate the NMDA-R hypofunction hypothesis.
Figures

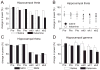
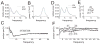

References
-
- Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156:1646–1649. - PubMed
-
- Baldeweg T, Spence S, Hirsch SR, Gruzelier J. Gamma-band electroencephalographic oscillations in a patient with somatic hallucinations. Lancet. 1998;352:620–621. - PubMed
-
- Becker A, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Test of predictive validity. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:1267–1277. - PubMed
-
- Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: A possible animal model of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2003;27:687–700. - PubMed
-
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

