Terminally differentiated astrocytes lack DNA damage response signaling and are radioresistant but retain DNA repair proficiency
- PMID: 21979466
- PMCID: PMC3307974
- DOI: 10.1038/cdd.2011.129
Terminally differentiated astrocytes lack DNA damage response signaling and are radioresistant but retain DNA repair proficiency
Abstract
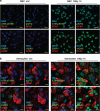
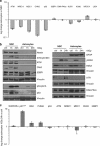
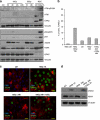
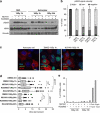
The impact and consequences of damage generation into genomic DNA, especially in the form of DNA double-strand breaks, and of the DNA-damage response (DDR) pathways that are promptly activated, have been elucidated in great detail. Most of this research, however, has been performed on proliferating, often cancerous, cell lines. In a mammalian body, the majority of cells are terminally differentiated (TD), and derives from a small pool of self-renewing somatic stem cells. Here, we comparatively studied DDR signaling and radiosensitivity in neural stem cells (NSC) and their TD-descendants, astrocytes - the predominant cells in the mammalian brain. Astrocytes have important roles in brain physiology, development and plasticity. We discovered that NSC activate canonical DDR upon exposure to ionizing radiation. Strikingly, astrocytes proved radioresistant, lacked functional DDR signaling, with key DDR genes such as ATM being repressed at the transcriptional level. Nevertheless, astrocytes retain the expression of non-homologous end-joining (NHEJ) genes and indeed they are DNA repair proficient. Unlike in NSC, in astrocytes DNA-PK seems to be the PI3K-like protein kinase responsible for γH2AX signal generation upon DNA damage. We also demonstrate the lack of functional DDR signaling activation in vivo in astrocytes of irradiated adult mouse brains, although adjacent neurons activate the DDR.
Figures
Similar articles
-
Multiple facets of the DNA damage response contribute to the radioresistance of mouse mesenchymal stromal cell lines.Stem Cells. 2013 Jan;31(1):137-45. doi: 10.1002/stem.1222. Stem Cells. 2013. PMID: 22961695
-
Dynamic dependence on ATR and ATM for double-strand break repair in human embryonic stem cells and neural descendants.PLoS One. 2010 Apr 2;5(4):e10001. doi: 10.1371/journal.pone.0010001. PLoS One. 2010. PMID: 20368801 Free PMC article.
-
Ionizing radiation induced signaling of DNA damage response molecules in RAW 264.7 and CD4⁺ T cells.Mol Cell Biochem. 2012 Apr;363(1-2):43-51. doi: 10.1007/s11010-011-1156-z. Epub 2011 Dec 16. Mol Cell Biochem. 2012. PMID: 22173400
-
The influence of heterochromatin on DNA double strand break repair: Getting the strong, silent type to relax.DNA Repair (Amst). 2010 Dec 10;9(12):1273-82. doi: 10.1016/j.dnarep.2010.09.013. Epub 2010 Oct 30. DNA Repair (Amst). 2010. PMID: 21036673 Review.
-
Involvement of DNA-PK and ATM in radiation- and heat-induced DNA damage recognition and apoptotic cell death.J Radiat Res. 2010;51(5):493-501. doi: 10.1269/jrr.10039. Epub 2010 Aug 28. J Radiat Res. 2010. PMID: 20814172 Review.
Cited by
-
Differential regulation of DNA damage response activation between somatic and germline cells in Caenorhabditis elegans.Cell Death Differ. 2012 Nov;19(11):1847-55. doi: 10.1038/cdd.2012.69. Epub 2012 Jun 15. Cell Death Differ. 2012. PMID: 22705849 Free PMC article.
-
Stem cells: the pursuit of genomic stability.Int J Mol Sci. 2014 Nov 14;15(11):20948-67. doi: 10.3390/ijms151120948. Int J Mol Sci. 2014. PMID: 25405730 Free PMC article. Review.
-
Regulatory coupling between long noncoding RNAs and senescence in irradiated microglia.J Neuroinflammation. 2020 Oct 28;17(1):321. doi: 10.1186/s12974-020-02001-1. J Neuroinflammation. 2020. PMID: 33109221 Free PMC article.
-
DNA repair kinetics in SCID mice Sertoli cells and DNA-PKcs-deficient mouse embryonic fibroblasts.Chromosoma. 2017 Mar;126(2):287-298. doi: 10.1007/s00412-016-0590-9. Epub 2016 May 2. Chromosoma. 2017. PMID: 27136939 Free PMC article.
-
Connecting Malfunctioning Glial Cells and Brain Degenerative Disorders.Genomics Proteomics Bioinformatics. 2016 Jun;14(3):155-165. doi: 10.1016/j.gpb.2016.04.003. Epub 2016 May 28. Genomics Proteomics Bioinformatics. 2016. PMID: 27245308 Free PMC article. Review.
References
-
- Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006;31:402–410. - PubMed
-
- d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. - PubMed
-
- Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. - PubMed
-
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

