TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway
- PMID: 21979919
- PMCID: PMC3197205
- DOI: 10.1101/gad.17224711
TNF-stimulated MAP kinase activation mediated by a Rho family GTPase signaling pathway
Abstract
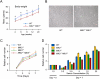
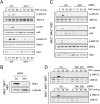
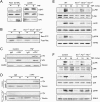
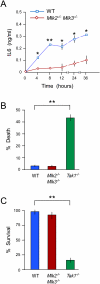
The biological response to tumor necrosis factor (TNF) involves activation of MAP kinases. Here we report a mechanism of MAP kinase activation by TNF that is mediated by the Rho GTPase family members Rac/Cdc42. This signaling pathway requires Src-dependent activation of the guanosine nucleotide exchange factor Vav, activation of Rac/Cdc42, and the engagement of the Rac/Cdc42 interaction site (CRIB motif) on mixed-lineage protein kinases (MLKs). We show that this pathway is essential for full MAP kinase activation during the response to TNF. Moreover, this MLK pathway contributes to inflammation in vivo.
Figures


References
-
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ 2000. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem 275: 10141–10149 - PubMed
-
- Bock BC, Vacratsis PO, Qamirani E, Gallo KA 2000. Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. J Biol Chem 275: 14231–14241 - PubMed
-
- Chadee DN, Kyriakis JM 2004. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol 6: 770–776 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
