Nucleophosmin interacts with FOXM1 and modulates the level and localization of FOXM1 in human cancer cells
- PMID: 21979956
- PMCID: PMC3308854
- DOI: 10.1074/jbc.M111.270843
Nucleophosmin interacts with FOXM1 and modulates the level and localization of FOXM1 in human cancer cells
Abstract
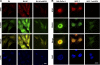
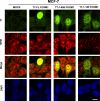
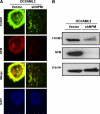
Using mass spectrometric analysis we found that oncogenic transcription factor FOXM1 that is overexpressed in a majority of human cancers interacts with multifunctional protein NPM, which is also overexpressed in a variety of human tumors. Coimmunoprecipitation and glutathione S-transferase pull-down experiments demonstrated that NPM forms a complex with FOXM1 and also identified the regions responsible for their interaction. Immunofluorescence microscopy confirmed the interaction between FOXM1 and NPM in cancer and immortal cells. Furthermore, knockdown of NPM in immortal and cancer cells led to significant down-regulation of FOXM1 similar to its levels in normal cells, suggesting that NPM might modulate FOXM1 level. In addition, in OCI/AML3 leukemia cells where mutant NPM is localized in the cytoplasm we found that typically nuclear FOXM1 was predominantly co-localized with NPM in the cytoplasm, while NPM knockdown led to the disappearance of FOXM1 from the cytoplasm, suggesting that NPM may also determine intracellular localization of FOXM1. Knockdown of FOXM1 or NPM in MIA PaCa-2 pancreatic cancer cells inhibited anchorage-dependent and independent growth in cell culture, and tumor growth in nude mice. In addition, over-expression of FOXM1 reversed the effect of NPM knockdown in vitro. Our data suggest that in cancer cells NPM interacts with FOXM1 and their interaction is required for sustaining the level and localization of FOXM1. Targeting the interaction between FOXM1 and NPM by peptides or small molecules may represent a novel therapeutic strategy against cancer.
Figures

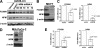
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

