Determinants of the spatiotemporal dynamics of the 2009 H1N1 pandemic in Europe: implications for real-time modelling
- PMID: 21980281
- PMCID: PMC3182874
- DOI: 10.1371/journal.pcbi.1002205
Determinants of the spatiotemporal dynamics of the 2009 H1N1 pandemic in Europe: implications for real-time modelling
Abstract
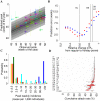
Influenza pandemics in the last century were characterized by successive waves and differences in impact and timing between different regions, for reasons not clearly understood. The 2009 H1N1 pandemic showed rapid global spread, but with substantial heterogeneity in timing within each hemisphere. Even within Europe substantial variation was observed, with the UK being unique in experiencing a major first wave of transmission in early summer and all other countries having a single major epidemic in the autumn/winter, with a West to East pattern of spread. Here we show that a microsimulation model, parameterised using data about H1N1pdm collected by the beginning of June 2009, explains the occurrence of two waves in UK and a single wave in the rest of Europe as a consequence of timing of H1N1pdm spread, fluxes of travels from US and Mexico, and timing of school vacations. The model provides a description of pandemic spread through Europe, depending on intra-European mobility patterns and socio-demographic structure of the European populations, which is in broad agreement with observed timing of the pandemic in different countries. Attack rates are predicted to depend on the socio-demographic structure, with age dependent attack rates broadly agreeing with available serological data. Results suggest that the observed heterogeneity can be partly explained by the between country differences in Europe: marked differences in school calendars, mobility patterns and sociodemographic structures. Moreover, higher susceptibility of children to infection played a key role in determining the epidemiology of the 2009 pandemic. Our work shows that it would have been possible to obtain a broad-brush prediction of timing of the European pandemic well before the autumn of 2009, much more difficult to achieve with simpler models or pre-pandemic parameterisation. This supports the use of models accounting for the structure of complex modern societies for giving insight to policy makers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

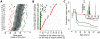
Similar articles
-
Age-specific contacts and travel patterns in the spatial spread of 2009 H1N1 influenza pandemic.BMC Infect Dis. 2013 Apr 15;13:176. doi: 10.1186/1471-2334-13-176. BMC Infect Dis. 2013. PMID: 23587010 Free PMC article.
-
Spatial Transmission of 2009 Pandemic Influenza in the US.PLoS Comput Biol. 2014 Jun 12;10(6):e1003635. doi: 10.1371/journal.pcbi.1003635. eCollection 2014 Jun. PLoS Comput Biol. 2014. PMID: 24921923 Free PMC article.
-
The community impact of the 2009 influenza pandemic in the WHO European region: a comparison with historical seasonal data from 28 countries.BMC Infect Dis. 2012 Feb 10;12:36. doi: 10.1186/1471-2334-12-36. BMC Infect Dis. 2012. PMID: 22325082 Free PMC article.
-
Historical and clinical aspects of the 1918 H1N1 pandemic in the United States.Virology. 2019 Jan 15;527:32-37. doi: 10.1016/j.virol.2018.10.019. Epub 2018 Nov 16. Virology. 2019. PMID: 30453209 Review.
-
Modelling during an emergency: the 2009 H1N1 influenza pandemic.Clin Microbiol Infect. 2013 Nov;19(11):1014-22. doi: 10.1111/1469-0691.12284. Epub 2013 Jun 25. Clin Microbiol Infect. 2013. PMID: 23800220 Review.
Cited by
-
The Scaling of Human Contacts and Epidemic Processes in Metapopulation Networks.Sci Rep. 2015 Oct 19;5:15111. doi: 10.1038/srep15111. Sci Rep. 2015. PMID: 26478209 Free PMC article.
-
Projecting social contact matrices in 152 countries using contact surveys and demographic data.PLoS Comput Biol. 2017 Sep 12;13(9):e1005697. doi: 10.1371/journal.pcbi.1005697. eCollection 2017 Sep. PLoS Comput Biol. 2017. PMID: 28898249 Free PMC article.
-
The role of different social contexts in shaping influenza transmission during the 2009 pandemic.Sci Rep. 2014 Nov 27;4:7218. doi: 10.1038/srep07218. Sci Rep. 2014. PMID: 25427621 Free PMC article.
-
Parameters for the mathematical modelling of Clostridium difficile acquisition and transmission: a systematic review.PLoS One. 2013 Dec 20;8(12):e84224. doi: 10.1371/journal.pone.0084224. eCollection 2013. PLoS One. 2013. PMID: 24376797 Free PMC article.
-
Reviewing the History of Pandemic Influenza: Understanding Patterns of Emergence and Transmission.Pathogens. 2016 Dec 6;5(4):66. doi: 10.3390/pathogens5040066. Pathogens. 2016. PMID: 27929449 Free PMC article. Review.
References
-
- Khan K, Arino J, Hu W, Raposo P, Sears J, et al. Spread of a Novel Influenza A (H1N1) Virus via Global Airline Transportation. N Engl J Med. 2009;361:212–214. - PubMed
-
- Hahné S, Donker T, Meijer A, Timen A, van Steenbergen J et al. Epidemiology and control of influenza A(H1N1)v in the Netherlands: the first 115 cases. Euro Surveill. 2009;14::pii = 19267. - PubMed
-
- Gilsdorf A, Poggensee G Working Group Pandemic Influenza A(H1N1)v. Influenza A(H1N1)v in Germany: the first 10,000 cases. Euro Surveill. 2009;14:pii = 19318. - PubMed
-
- Belgian working group on influenza A(H1N1)v. Influenza A(H1N1)v virus infections in Belgium. Euro Surveill. 2009;14:pii = 19270. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

