Genomic analysis of QTLs and genes altering natural variation in stochastic noise
- PMID: 21980300
- PMCID: PMC3183082
- DOI: 10.1371/journal.pgen.1002295
Genomic analysis of QTLs and genes altering natural variation in stochastic noise
Abstract
Quantitative genetic analysis has long been used to study how natural variation of genotype can influence an organism's phenotype. While most studies have focused on genetic determinants of phenotypic average, it is rapidly becoming understood that stochastic noise is genetically determined. However, it is not known how many traits display genetic control of stochastic noise nor how broadly these stochastic loci are distributed within the genome. Understanding these questions is critical to our understanding of quantitative traits and how they relate to the underlying causal loci, especially since stochastic noise may be directly influenced by underlying changes in the wiring of regulatory networks. We identified QTLs controlling natural variation in stochastic noise of glucosinolates, plant defense metabolites, as well as QTLs for stochastic noise of related transcripts. These loci included stochastic noise QTLs unique for either transcript or metabolite variation. Validation of these loci showed that genetic polymorphism within the regulatory network alters stochastic noise independent of effects on corresponding average levels. We examined this phenomenon more globally, using transcriptomic datasets, and found that the Arabidopsis transcriptome exhibits significant, heritable differences in stochastic noise. Further analysis allowed us to identify QTLs that control genomic stochastic noise. Some genomic QTL were in common with those altering average transcript abundance, while others were unique to stochastic noise. Using a single isogenic population, we confirmed that natural variation at ELF3 alters stochastic noise in the circadian clock and metabolism. Since polymorphisms controlling stochastic noise in genomic phenotypes exist within wild germplasm for naturally selected phenotypes, this suggests that analysis of Arabidopsis evolution should account for genetic control of stochastic variance and average phenotypes. It remains to be determined if natural genetic variation controlling stochasticity is equally distributed across the genomes of other multi-cellular eukaryotes.
Conflict of interest statement
The authors have declared that no competing interests exist.
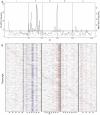
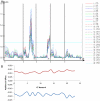
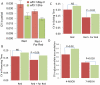
Figures

References
-
- Falconer DS, Mackay TFC. Essex: Longman, Harlow; 1996. Introduction to Quantitative Genetics.
-
- Lynch M, Walsh B. Sunderland, Massachusetts: Sinauer Associates, Inc; 1998. Genetics and analysis of quantitative traits.
-
- Zhang X, Shiu S, Cal A, Borevitz JO. Global analysis of genetic, epigenetic and transcriptional polymorphisms in Arabidopsis thaliana using whole genome tiling arrays. PLoS Genet. 2008;4:e1000032. doi: 10.1371/journal.pgen.1000032. - DOI - PMC - PubMed
-
- Albert R, Barabasi AL. Statistical mechanics of complex networks. Reviews of Modern Physics. 2002;74:47–97.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

