Genome-wide analysis of heteroduplex DNA in mismatch repair-deficient yeast cells reveals novel properties of meiotic recombination pathways
- PMID: 21980306
- PMCID: PMC3183076
- DOI: 10.1371/journal.pgen.1002305
Genome-wide analysis of heteroduplex DNA in mismatch repair-deficient yeast cells reveals novel properties of meiotic recombination pathways
Abstract
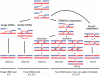
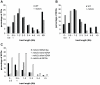
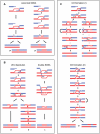
Meiotic DNA double-strand breaks (DSBs) initiate crossover (CO) recombination, which is necessary for accurate chromosome segregation, but DSBs may also repair as non-crossovers (NCOs). Multiple recombination pathways with specific intermediates are expected to lead to COs and NCOs. We revisited the mechanisms of meiotic DSB repair and the regulation of CO formation, by conducting a genome-wide analysis of strand-transfer intermediates associated with recombination events. We performed this analysis in a SK1 × S288C Saccharomyces cerevisiae hybrid lacking the mismatch repair (MMR) protein Msh2, to allow efficient detection of heteroduplex DNAs (hDNAs). First, we observed that the anti-recombinogenic activity of MMR is responsible for a 20% drop in CO number, suggesting that in MMR-proficient cells some DSBs are repaired using the sister chromatid as a template when polymorphisms are present. Second, we observed that a large fraction of NCOs were associated with trans-hDNA tracts constrained to a single chromatid. This unexpected finding is compatible with dissolution of double Holliday junctions (dHJs) during repair, and it suggests the existence of a novel control point for CO formation at the level of the dHJ intermediate, in addition to the previously described control point before the dHJ formation step. Finally, we observed that COs are associated with complex hDNA patterns, confirming that the canonical double-strand break repair model is not sufficient to explain the formation of most COs. We propose that multiple factors contribute to the complexity of recombination intermediates. These factors include repair of nicks and double-stranded gaps, template switches between non-sister and sister chromatids, and HJ branch migration. Finally, the good correlation between the strand transfer properties observed in the absence of and in the presence of Msh2 suggests that the intermediates detected in the absence of Msh2 reflect normal intermediates.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Eliminating both canonical and short-patch mismatch repair in Drosophila melanogaster suggests a new meiotic recombination model.PLoS Genet. 2014 Sep 4;10(9):e1004583. doi: 10.1371/journal.pgen.1004583. eCollection 2014 Sep. PLoS Genet. 2014. PMID: 25188408 Free PMC article.
-
Double Holliday junctions are intermediates of DNA break repair.Nature. 2010 Apr 8;464(7290):937-41. doi: 10.1038/nature08868. Epub 2010 Mar 28. Nature. 2010. PMID: 20348905 Free PMC article.
-
Mechanistic Insight into Crossing over during Mouse Meiosis.Mol Cell. 2020 Jun 18;78(6):1252-1263.e3. doi: 10.1016/j.molcel.2020.04.009. Epub 2020 May 1. Mol Cell. 2020. PMID: 32362315 Free PMC article.
-
Meiotic versus mitotic recombination: two different routes for double-strand break repair: the different functions of meiotic versus mitotic DSB repair are reflected in different pathway usage and different outcomes.Bioessays. 2010 Dec;32(12):1058-66. doi: 10.1002/bies.201000087. Epub 2010 Oct 21. Bioessays. 2010. PMID: 20967781 Free PMC article. Review.
-
New and old ways to control meiotic recombination.Trends Genet. 2011 Oct;27(10):411-21. doi: 10.1016/j.tig.2011.06.007. Epub 2011 Jul 21. Trends Genet. 2011. PMID: 21782271 Free PMC article. Review.
Cited by
-
Association of Nurr1 gene mutations with Parkinson's disease in the Han population living in the Hubei province of China.Neural Regen Res. 2012 Aug 15;7(23):1791-6. doi: 10.3969/j.issn.1673-5374.2012.23.005. Neural Regen Res. 2012. PMID: 25624803 Free PMC article.
-
High-Throughput Analysis of Heteroduplex DNA in Mitotic Recombination Products.Methods Mol Biol. 2021;2153:503-519. doi: 10.1007/978-1-0716-0644-5_34. Methods Mol Biol. 2021. PMID: 32840801 Free PMC article.
-
GC-biased gene conversion in yeast is specifically associated with crossovers: molecular mechanisms and evolutionary significance.Mol Biol Evol. 2013 Jun;30(6):1409-19. doi: 10.1093/molbev/mst056. Epub 2013 Mar 16. Mol Biol Evol. 2013. PMID: 23505044 Free PMC article.
-
Kinetochores, cohesin, and DNA breaks: Controlling meiotic recombination within pericentromeres.Yeast. 2019 Mar;36(3):121-127. doi: 10.1002/yea.3366. Epub 2019 Feb 3. Yeast. 2019. PMID: 30625250 Free PMC article. Review.
-
Repair of DNA double-strand breaks in plant meiosis: role of eukaryotic RecA recombinases and their modulators.Plant Reprod. 2023 Mar;36(1):17-41. doi: 10.1007/s00497-022-00443-6. Epub 2022 Jun 1. Plant Reprod. 2023. PMID: 35641832 Review.
References
-
- Page SL, Hawley RS. Chromosome choreography: the meiotic ballet. Science. 2003;301:785–789. - PubMed
-
- Arora C, Kee K, Maleki S, Keeney S. Antiviral protein Ski8 is a direct partner of Spo11 in meiotic DNA break formation, independent of its cytoplasmic role in RNA metabolism. Mol Cell. 2004;13:549–559. - PubMed
-
- Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, et al. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. - PubMed
-
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

