Lymnaea schirazensis, an overlooked snail distorting fascioliasis data: genotype, phenotype, ecology, worldwide spread, susceptibility, applicability
- PMID: 21980347
- PMCID: PMC3183092
- DOI: 10.1371/journal.pone.0024567
Lymnaea schirazensis, an overlooked snail distorting fascioliasis data: genotype, phenotype, ecology, worldwide spread, susceptibility, applicability
Abstract
Background: Lymnaeid snails transmit medical and veterinary important trematodiases, mainly fascioliasis. Vector specificity of fasciolid parasites defines disease distribution and characteristics. Different lymnaeid species appear linked to different transmission and epidemiological patterns. Pronounced susceptibility differences to absolute resistance have been described among lymnaeid populations. When assessing disease characteristics in different endemic areas, unexpected results were obtained in studies on lymnaeid susceptibility to Fasciola. We undertook studies to understand this disease transmission heterogeneity.
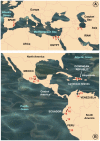
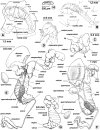
Methodology/principal findings: A ten-year study in Iran, Egypt, Spain, the Dominican Republic, Mexico, Venezuela, Ecuador and Peru, demonstrated that such heterogeneity is not due to susceptibility differences, but to a hitherto overlooked cryptic species, Lymnaea schirazensis, confused with the main vector Galba truncatula and/or other Galba/Fossaria vectors. Nuclear rDNA and mtDNA sequences and phylogenetic reconstruction highlighted an old evolutionary divergence from other Galba/Fossaria species, and a low intraspecific variability suggesting a recent spread from one geographical source. Morphometry, anatomy and egg cluster analyses allowed for phenotypic differentiation. Selfing, egg laying, and habitat characteristics indicated a migration capacity by passive transport. Studies showed that it is not a vector species (n = 8572 field collected, 20 populations): snail finding and penetration by F. hepatica miracidium occur but never lead to cercarial production (n = 338 experimentally infected).
Conclusions/significance: This species has been distorting fasciolid specificity/susceptibility and fascioliasis geographical distribution data. Hence, a large body of literature on G. truncatula should be revised. Its existence has henceforth to be considered in research. Genetic data on livestock, archeology and history along the 10,000-year post-domestication period explain its wide spread from the Neolithic Fertile Crescent. It is an efficient biomarker for the follow-up of livestock movements, a crucial aspect in fascioliasis emergence. It offers an outstanding laboratory model for genetic studies on susceptibility/resistance in F. hepatica/lymnaeid interaction, a field of applied research with disease control perspectives.
Conflict of interest statement
Figures












References
-
- Bargues MD, Vigo M, Horak P, Dvorak J, Patzner RA, et al. European Lymnaeidae (Mollusca: Gastropoda), intermediate hosts of trematodiases, based on nuclear ribosomal DNA ITS-2 sequences. Infect Genet Evol. 2001;1:85–107. - PubMed
-
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005;35:1255–1278. - PubMed
-
- World Health Organization. Control of foodborne trematode infections. WHO Technical Report Series. 1995;849:1–157. - PubMed
-
- Mas-Coma S, Valero MA, Bargues MD. Effects of climate change on animal and zoonotic helminthiases. Rev Sci Tech. 2008;27:443–457. - PubMed
-
- Mas-Coma S, Valero MA, Bargues MD. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet Parasitol. 2009;163:264–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

