Intravascular food reward
- PMID: 21980372
- PMCID: PMC3181252
- DOI: 10.1371/journal.pone.0024992
Intravascular food reward
Abstract
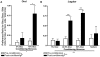
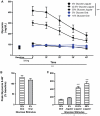
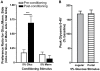
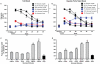
Consumption of calorie-containing sugars elicits appetitive behavioral responses and dopamine release in the ventral striatum, even in the absence of sweet-taste transduction machinery. However, it is unclear if such reward-related postingestive effects reflect preabsorptive or postabsorptive events. In support of the importance of postabsorptive glucose detection, we found that, in rat behavioral tests, high concentration glucose solutions administered in the jugular vein were sufficient to condition a side-bias. Additionally, a lower concentration glucose solution conditioned robust behavioral responses when administered in the hepatic-portal, but not the jugular vein. Furthermore, enteric administration of glucose at a concentration that is sufficient to elicit behavioral conditioning resulted in a glycemic profile similar to that observed after administration of the low concentration glucose solution in the hepatic-portal, but not jugular vein. Finally using fast-scan cyclic voltammetry we found that, in accordance with behavioral findings, a low concentration glucose solution caused an increase in spontaneous dopamine release events in the nucleus accumbens shell when administered in the hepatic-portal, but not the jugular vein. These findings demonstrate that the postabsorptive effects of glucose are sufficient for the postingestive behavioral and dopaminergic reward-related responses that result from sugar consumption. Furthermore, glycemia levels in the hepatic-portal venous system contribute more significantly for this effect than systemic glycemia, arguing for the participation of an intra-abdominal visceral sensor for glucose.
Conflict of interest statement
Figures
References
-
- Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. - PubMed
-
- de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, et al. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. - PubMed
-
- Frank GK, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, et al. Sucrose activates human taste pathways differently from artificial sweetener. Neuroimage. 2008;39:1559–1569. - PubMed
-
- Touzani K, Bodnar R, Sclafani A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur J Neurosci. 2008;27:1525–1533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

