Fructose modulates cardiomyocyte excitation-contraction coupling and Ca²⁺ handling in vitro
- PMID: 21980397
- PMCID: PMC3182977
- DOI: 10.1371/journal.pone.0025204
Fructose modulates cardiomyocyte excitation-contraction coupling and Ca²⁺ handling in vitro
Abstract
Background: High dietary fructose has structural and metabolic cardiac impact, but the potential for fructose to exert direct myocardial action is uncertain. Cardiomyocyte functional responsiveness to fructose, and capacity to transport fructose has not been previously demonstrated.
Objective: The aim of the present study was to seek evidence of fructose-induced modulation of cardiomyocyte excitation-contraction coupling in an acute, in vitro setting.
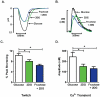
Methods and results: The functional effects of fructose on isolated adult rat cardiomyocyte contractility and Ca²⁺ handling were evaluated under physiological conditions (37°C, 2 mM Ca²⁺, HEPES buffer, 4 Hz stimulation) using video edge detection and microfluorimetry (Fura2) methods. Compared with control glucose (11 mM) superfusate, 2-deoxyglucose (2 DG, 11 mM) substitution prolonged both the contraction and relaxation phases of the twitch (by 16 and 36% respectively, p<0.05) and this effect was completely abrogated with fructose supplementation (11 mM). Similarly, fructose prevented the Ca²⁺ transient delay induced by exposure to 2 DG (time to peak Ca²⁺ transient: 2 DG: 29.0±2.1 ms vs. glucose: 23.6±1.1 ms vs. fructose +2 DG: 23.7±1.0 ms; p<0.05). The presence of the fructose transporter, GLUT5 (Slc2a5) was demonstrated in ventricular cardiomyocytes using real time RT-PCR and this was confirmed by conventional RT-PCR.
Conclusion: This is the first demonstration of an acute influence of fructose on cardiomyocyte excitation-contraction coupling. The findings indicate cardiomyocyte capacity to transport and functionally utilize exogenously supplied fructose. This study provides the impetus for future research directed towards characterizing myocardial fructose metabolism and understanding how long term high fructose intake may contribute to modulating cardiac function.
Conflict of interest statement
Figures



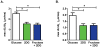
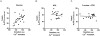

References
-
- Mellor KM, Ritchie RH, Delbridge LM. Reactive oxygen species and insulin-resistant cardiomyopathy. Clin Exp Pharmacol Physiol. 2010;37:222–228. - PubMed
-
- Mellor KM, Ritchie RH, Davidoff AJ, Delbridge LMD. Elevated dietary sugar and the heart: experimental models and myocardial remodeling. Can J Physiol Pharmacol. 2010;88:525–540. - PubMed
-
- Henry RR, Crapo PA, Thorburn AW. Current issues in fructose metabolism. Annu Rev Nutr. 1991;11:21–39. - PubMed
-
- Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

