Obesity risk gene TMEM18 encodes a sequence-specific DNA-binding protein
- PMID: 21980424
- PMCID: PMC3182218
- DOI: 10.1371/journal.pone.0025317
Obesity risk gene TMEM18 encodes a sequence-specific DNA-binding protein
Abstract
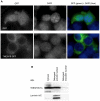
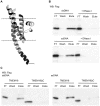
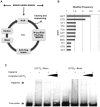
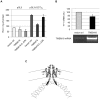
Transmembrane protein 18 (TMEM18) has previously been connected to cell migration and obesity. However, the molecular function of the protein has not yet been described. Here we show that TMEM18 localises to the nuclear membrane and binds to DNA in a sequence-specific manner. The protein binds DNA with its positively charged C-terminus that contains also a nuclear localisation signal. Increase in the amount of TMEM18 in cells suppresses expression from a reporter vector with the TMEM18 target sequence. TMEM18 is a small protein of 140 residues and is predicted to be mostly alpha-helical with three transmembrane parts. As a consequence the DNA binding by TMEM18 would bring the chromatin very near to nuclear membrane. We speculate that this closed perinuclear localisation of TMEM18-bound DNA might repress transcription from it.
Conflict of interest statement
Figures

References
-
- Jurvansuu J, Zhao Y, Leung DS, Boulaire J, Yu YH, et al. Transmembrane protein 18 enhances the tropism of neural stem cells for glioma cells. Cancer Res. 2008;68:4614–4622. - PubMed
-
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

