Activation of WNT/β-catenin signaling in pulmonary fibroblasts by TGF-β₁ is increased in chronic obstructive pulmonary disease
- PMID: 21980461
- PMCID: PMC3184127
- DOI: 10.1371/journal.pone.0025450
Activation of WNT/β-catenin signaling in pulmonary fibroblasts by TGF-β₁ is increased in chronic obstructive pulmonary disease
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterized by abnormal extracellular matrix (ECM) turnover. Recently, activation of the WNT/β-catenin pathway has been associated with abnormal ECM turnover in various chronic diseases. We determined WNT-pathway gene expression in pulmonary fibroblasts of individuals with and without COPD and disentangled the role of β-catenin in fibroblast phenotype and function.
Methods: We assessed the expression of WNT-pathway genes and the functional role of β-catenin, using MRC-5 human lung fibroblasts and primary pulmonary fibroblasts of individuals with and without COPD.
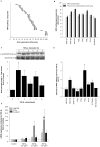
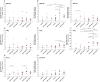
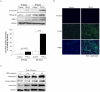
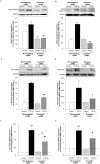
Results: Pulmonary fibroblasts expressed mRNA of genes required for WNT signaling. Stimulation of fibroblasts with TGF-β₁, a growth factor important in COPD pathogenesis, induced WNT-5B, FZD₈, DVL3 and β-catenin mRNA expression. The induction of WNT-5B, FZD₆, FZD₈ and DVL3 mRNA by TGF-β₁ was higher in fibroblasts of individuals with COPD than without COPD, whilst basal expression was similar. Accordingly, TGF-β₁ activated β-catenin signaling, as shown by an increase in transcriptionally active and total β-catenin protein expression. Furthermore, TGF-β₁induced the expression of collagen1α1, α-sm-actin and fibronectin, which was attenuated by β-catenin specific siRNA and by pharmacological inhibition of β-catenin, whereas the TGF-β₁-induced expression of PAI-1 was not affected. The induction of transcriptionally active β-catenin and subsequent fibronectin deposition induced by TGF-β₁ were enhanced in pulmonary fibroblasts from individuals with COPD.
Conclusions: β-catenin signaling contributes to ECM production by pulmonary fibroblasts and contributes to myofibroblasts differentiation. WNT/β-catenin pathway expression and activation by TGF-β₁ is enhanced in pulmonary fibroblasts from individuals with COPD. This suggests an important role of the WNT/β-catenin pathway in regulating fibroblast phenotype and function in COPD.
Conflict of interest statement
Figures


References
-
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. - PubMed
-
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. - PubMed
-
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22:672–688. - PubMed
-
- Konigshoff M, Kneidinger N, Eickelberg O. TGF-beta signaling in COPD: deciphering genetic and cellular susceptibilities for future therapeutic regimen. Swiss Med Wkly. 2009;139:554–563. - PubMed
-
- Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J. 2001;(Suppl 34):50s–59s. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

