Immunoprotectivity of HLA-A2 CTL peptides derived from respiratory syncytial virus fusion protein in HLA-A2 transgenic mouse
- PMID: 21980478
- PMCID: PMC3183052
- DOI: 10.1371/journal.pone.0025500
Immunoprotectivity of HLA-A2 CTL peptides derived from respiratory syncytial virus fusion protein in HLA-A2 transgenic mouse
Abstract
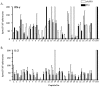
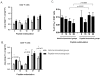
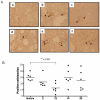
Identification of HLA-restricted CD8+ T cell epitopes is important to study RSV-induced immunity and illness. We algorithmically analyzed the sequence of the fusion protein (F) of respiratory syncytial virus (RSV) and generated synthetic peptides that can potentially bind to HLA-A*0201. Four out of the twenty-five 9-mer peptides tested: peptides 3 (F33-41), 13 (F214-222), 14 (F273-281), and 23 (F559-567), were found to bind to HLA-A*0201 with moderate to high affinity and were capable of inducing IFN-γ and IL-2 secretion in lymphocytes from HLA-A*0201 transgenic (HLA-Tg) mice pre-immunized with RSV or recombinant adenovirus expressing RSV F. HLA-Tg mice were immunized with these four peptides and were found to induce both Th1 and CD8+ T cell responses in in vitro secondary recall. Effector responses induced by these peptides were observed to confer differential protection against live RSV challenge. These peptides also caused better recovery of body weight loss induced by RSV. A significant reduction of lung viral load was observed in mice immunized with peptide 23, which appeared to enhance the levels of inflammatory chemokines (CCL17, CCL22, and IL-18) but did not increase eosinophil infiltration in the lungs. Whereas, significant reduction of infiltrated eosinophils induced by RSV infection was found in mice pre-immunized with peptide 13. Our results suggest that HLA-A2-restricted epitopes of RSV F protein could be useful for the development of epitope-based RSV vaccine.
Conflict of interest statement
Figures
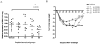


Similar articles
-
Human immunodeficiency virus type 1 Nef epitopes recognized in HLA-A2 transgenic mice in response to DNA and peptide immunization.Virology. 2000 Jul 20;273(1):112-9. doi: 10.1006/viro.2000.0360. Virology. 2000. PMID: 10891413
-
Virus-specific CTL responses induced by an H-2K(d)-restricted, motif-negative 15-mer peptide from the fusion protein of respiratory syncytial virus.J Gen Virol. 2002 Feb;83(Pt 2):429-438. doi: 10.1099/0022-1317-83-2-429. J Gen Virol. 2002. PMID: 11807236
-
Pools of lipidated HTL-CTL constructs prime for multiple HBV and HCV CTL epitope responses.Vaccine. 1998 May;16(8):823-33. doi: 10.1016/s0264-410x(97)00264-8. Vaccine. 1998. PMID: 9627940
-
Protein Crystallography in Vaccine Research and Development.Int J Mol Sci. 2015 Jun 9;16(6):13106-40. doi: 10.3390/ijms160613106. Int J Mol Sci. 2015. PMID: 26068237 Free PMC article. Review.
-
Max Bergmann lecture protein epitope mimetics in the age of structural vaccinology.J Pept Sci. 2013 Mar;19(3):127-40. doi: 10.1002/psc.2482. Epub 2013 Jan 24. J Pept Sci. 2013. PMID: 23349031 Free PMC article. Review.
Cited by
-
Respiratory syncytial virus--a comprehensive review.Clin Rev Allergy Immunol. 2013 Dec;45(3):331-79. doi: 10.1007/s12016-013-8368-9. Clin Rev Allergy Immunol. 2013. PMID: 23575961 Free PMC article. Review.
-
Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections.Mol Ther. 2012 Aug;20(8):1622-32. doi: 10.1038/mt.2012.130. Epub 2012 Jul 17. Mol Ther. 2012. PMID: 22801446 Free PMC article.
-
Bivalent vaccines effectively protect mice against influenza A and respiratory syncytial viruses.Emerg Microbes Infect. 2023 Dec;12(1):2192821. doi: 10.1080/22221751.2023.2192821. Emerg Microbes Infect. 2023. PMID: 36927227 Free PMC article.
-
Improved mRNA-based RSV vaccine with PreF forming enveloped virus-like particles.NPJ Vaccines. 2025 Jul 12;10(1):152. doi: 10.1038/s41541-025-01205-x. NPJ Vaccines. 2025. PMID: 40651980 Free PMC article.
-
Protective and dysregulated T cell immunity in RSV infection.Curr Opin Virol. 2013 Aug;3(4):468-74. doi: 10.1016/j.coviro.2013.05.005. Epub 2013 Jun 25. Curr Opin Virol. 2013. PMID: 23806514 Free PMC article. Review.
References
-
- Falsey AR. Respiratory syncytial virus infection in elderly and high-risk adults. Exp Lung Res. 2005;31(Suppl 1):77. - PubMed
-
- Holzel A, Parker L, Patterson WH, White LL, Thompson KM, et al. The isolation of respiratory syncytial virus from children with acute respiratory disease. Lancet. 1963;1:295–298. - PubMed
-
- Chanock RFL. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II. Epidemiologic aspects of infection in infants and young children. Am J Hyg. 1957;66:291–300. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

