Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man
- PMID: 21980503
- PMCID: PMC3182238
- DOI: 10.1371/journal.pone.0025612
Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man
Abstract
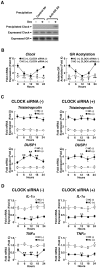
Context and objective: Circulating cortisol fluctuates diurnally under the control of the "master" circadian CLOCK, while the peripheral "slave" counterpart of the latter regulates the transcriptional activity of the glucocorticoid receptor (GR) at local glucocorticoid target tissues through acetylation. In this manuscript, we studied the effect of CLOCK-mediated GR acetylation on the sensitivity of peripheral tissues to glucocorticoids in humans.
Design and participants: We examined GR acetylation and mRNA expression of GR, CLOCK-related and glucocorticoid-responsive genes in peripheral blood mononuclear cells (PBMCs) obtained at 8 am and 8 pm from 10 healthy subjects, as well as in PBMCs obtained in the morning and cultured for 24 hours with exposure to 3-hour hydrocortisone pulses every 6 hours. We used EBV-transformed lymphocytes (EBVLs) as non-synchronized controls.
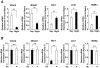
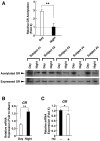
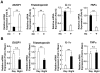
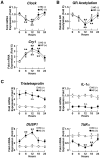
Results: GR acetylation was higher in the morning than in the evening in PBMCs, mirroring the fluctuations of circulating cortisol in reverse phase. All known glucocorticoid-responsive genes tested responded as expected to hydrocortisone in non-synchronized EBVLs, however, some of these genes did not show the expected diurnal mRNA fluctuations in PBMCs in vivo. Instead, their mRNA oscillated in a Clock- and a GR acetylation-dependent fashion in naturally synchronized PBMCs cultured ex vivo in the absence of the endogenous glucocorticoid, suggesting that circulating cortisol might prevent circadian GR acetylation-dependent effects in some glucocorticoid-responsive genes in vivo.
Conclusions: Peripheral CLOCK-mediated circadian acetylation of the human GR may function as a target-tissue, gene-specific counter regulatory mechanism to the actions of diurnally fluctuating cortisol, effectively decreasing tissue sensitivity to glucocorticoids in the morning and increasing it at night.
Conflict of interest statement
Figures

Similar articles
-
Circadian rhythms of glucocorticoid hormone actions in target tissues: potential clinical implications.Sci Signal. 2012 Oct 2;5(244):pt4. doi: 10.1126/scisignal.2003333. Sci Signal. 2012. PMID: 23033538 Free PMC article.
-
Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications.FASEB J. 2009 May;23(5):1572-83. doi: 10.1096/fj.08-117697. Epub 2009 Jan 13. FASEB J. 2009. PMID: 19141540 Free PMC article.
-
Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis.Neurogastroenterol Motil. 2016 Jan;28(1):12-25. doi: 10.1111/nmo.12706. Neurogastroenterol Motil. 2016. PMID: 26690871 Free PMC article. Review.
-
Acetylation-mediated epigenetic regulation of glucocorticoid receptor activity: circadian rhythm-associated alterations of glucocorticoid actions in target tissues.Mol Cell Endocrinol. 2011 Apr 10;336(1-2):23-30. doi: 10.1016/j.mce.2010.12.001. Epub 2010 Dec 10. Mol Cell Endocrinol. 2011. PMID: 21146585 Free PMC article.
-
Cardio-metabolic consequences of glucocorticoid replacement: relevance of ultradian signalling.Clin Endocrinol (Oxf). 2014 May;80(5):621-8. doi: 10.1111/cen.12422. Epub 2014 Feb 25. Clin Endocrinol (Oxf). 2014. PMID: 24611992 Review.
Cited by
-
How might circadian rhythms control mood? Let me count the ways..Biol Psychiatry. 2013 Aug 15;74(4):242-9. doi: 10.1016/j.biopsych.2013.02.019. Epub 2013 Apr 1. Biol Psychiatry. 2013. PMID: 23558300 Free PMC article. Review.
-
Glucocorticoids and the regulation of growth hormone secretion.Nat Rev Endocrinol. 2013 May;9(5):265-76. doi: 10.1038/nrendo.2013.5. Epub 2013 Feb 5. Nat Rev Endocrinol. 2013. PMID: 23381030 Review.
-
The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease.J Allergy Clin Immunol. 2013 Nov;132(5):1033-44. doi: 10.1016/j.jaci.2013.09.007. Epub 2013 Sep 29. J Allergy Clin Immunol. 2013. PMID: 24084075 Free PMC article. Review.
-
The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids.Endocr Rev. 2017 Feb 1;38(1):3-45. doi: 10.1210/er.2015-1080. Endocr Rev. 2017. PMID: 27749086 Free PMC article. Review.
-
Clocks, metabolism, and the epigenome.Mol Cell. 2012 Jul 27;47(2):158-67. doi: 10.1016/j.molcel.2012.06.026. Mol Cell. 2012. PMID: 22841001 Free PMC article. Review.
References
-
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–277. - PubMed
-
- Hastings M, O'Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. - PubMed
-
- Kalsbeek A, Palm IF, La Fleur SE, Scheer FA, Perreau-Lenz S, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21:458–469. - PubMed
-
- Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

