Excitotoxicity triggered by Neurobasal culture medium
- PMID: 21980512
- PMCID: PMC3182245
- DOI: 10.1371/journal.pone.0025633
Excitotoxicity triggered by Neurobasal culture medium
Abstract
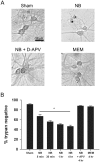
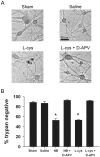
Neurobasal defined culture medium has been optimized for survival of rat embryonic hippocampal neurons and is now widely used for many types of primary neuronal cell culture. Therefore, we were surprised that routine medium exchange with serum- and supplement-free Neurobasal killed as many as 50% of postnatal hippocampal neurons after a 4 h exposure at day in vitro 12-15. Minimal Essential Medium (MEM), in contrast, produced no significant toxicity. Detectable Neurobasal-induced neuronal death occurred with as little as 5 min exposure, measured 24 h later. D-2-Amino-5-phosphonovalerate (D-APV) completely prevented Neurobasal toxicity, implicating direct or indirect N-methyl-D-aspartate (NMDA) receptor-mediated neuronal excitotoxicity. Whole-cell recordings revealed that Neurobasal but not MEM directly activated D-APV-sensitive currents similar in amplitude to those gated by 1 µM glutamate. We hypothesized that L-cysteine likely mediates the excitotoxic effects of Neurobasal incubation. Although the original published formulation of Neurobasal contained only 10 µM L-cysteine, commercial recipes contain 260 µM, a concentration in the range reported to activate NMDA receptors. Consistent with our hypothesis, 260 µM L-cysteine in bicarbonate-buffered saline gated NMDA receptor currents and produced toxicity equivalent to Neurobasal. Although NMDA receptor-mediated depolarization and Ca²⁺ influx may support survival of young neurons, NMDA receptor agonist effects on development and survival should be considered when employing Neurobasal culture medium.
Conflict of interest statement
Figures


References
-
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. - PubMed
-
- Olney JW, Zorumski C, Price MT, Labruyere J. L-cysteine, a bicarbonate-sensitive endogenous excitotoxin. Science. 1990;248:596–599. - PubMed
-
- Shute AA, Cormier RJ, Moulder KL, Benz A, Isenberg KE, et al. Astrocytes exert a pro-apoptotic effect on neurons in postnatal hippocampal cultures. Neuroscience. 2005;131:349–358. - PubMed
-
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

